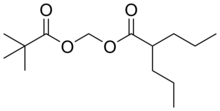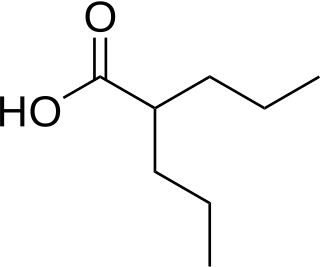
Valproate (VPA) and its valproic acid, sodium valproate, and valproate semisodium forms are medications primarily used to treat epilepsy and bipolar disorder and prevent migraine headaches. They are useful for the prevention of seizures in those with absence seizures, partial seizures, and generalized seizures. They can be given intravenously or by mouth, and the tablet forms exist in both long- and short-acting formulations.

Valpromide is a carboxamide derivative of valproic acid used in the treatment of epilepsy and some affective disorders. It is rapidly metabolised (80%) to valproic acid but has anticonvulsant properties itself. It may produce more stable plasma levels than valproic acid or sodium valproate and may be more effective at preventing febrile seizures. However, it is over one hundred times more potent as an inhibitor of liver microsomal epoxide hydrolase. This makes it incompatible with carbamazepine and can affect the ability of the body to remove other toxins. Valpromide is no safer during pregnancy than valproic acid.

Cortisone acetate is a synthetic glucocorticoid corticosteroid and corticosteroid ester which is marketed in many countries throughout the world, including in the United States, the United Kingdom, and various other European countries. It is the C21 acetate ester of cortisone, and acts as a prodrug of cortisone in the body.

Flumetasone, also known as flumethasone, is a corticosteroid for topical use.
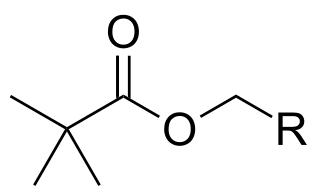
Pivaloyloxymethyl (POM, pivoxil, pivoxyl) is a protecting group used in organic synthesis. The POM radical has the formula (CH3)3C-CO-O-CH2.

Aceburic acid (INN), also known as 4-acetoxybutanoic acid or 4-hydroxybutyric acid acetate, is drug described as an analgesic which was never marketed. It is the acetyl ester of gamma-hydroxybutyrate, and based on its structural relation to GHB, is likely to behave as a prodrug to it.

Clocortolone pivalate, also known as clocortolone trimethylacetate, is a synthetic glucocorticoid corticosteroid and corticosteroid ester which is marketed in the United States and Austria. It is the C21 pivalate (trimethylacetate) ester of clocortolone, and acts as a prodrug of clocortolone in the body.

Stenbolone is an anabolic–androgenic steroid (AAS) of the dihydrotestosterone (DHT) group which was never marketed. A C17β ester prodrug of stenbolone, stenbolone acetate, is used as an AAS for depot intramuscular injection under the brand names Anatrofin and Stenobolone.
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability. In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat. Conversely, this is not the case with intravenous injection or oral administration. Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot. Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen.

Diacetylnalorphine (BAN) is an opioid drug described as an analgesic and antidote which was never marketed. It is the 3,6-diacetyl ester of nalorphine, and therefore the heroin analogue of nalorphine. Diacetylnalorphine may behave as a prodrug to nalorphine, similarly to the cases of heroin (diacetylmorphine) to morphine and diacetyldihydromorphine to dihydromorphine.

A GABA analogue is a compound which is an analogue or derivative of the neurotransmitter gamma-Aminobutyric acid (GABA).

Androstanolone benzoate, also known as stanolone benzoate or dihydrotestosterone benzoate (DHTB), as well as 5α-androstan-17β-ol-3-one 17β-benzoate, is a synthetic androgen and anabolic steroid and a dihydrotestosterone ester. It is used as an injectable and acts as a prodrug of androstanolone.

Androstanolone propionate, also known as stanolone propionate or dihydrotestosterone propionate (DHTP), as well as 5α-androstan-17β-ol-3-one 17β-propionate, is a synthetic androgen and anabolic steroid and a dihydrotestosterone ester that is marketed in Italy. It is used as an injectable and acts as a prodrug of androstanolone.

Androstanolone valerate, also known as stanolone valerate or dihydrotestosterone pentanoate, as well as 5α-androstan-17β-3-one 17β-valerate, is a synthetic androgen and anabolic steroid and a dihydrotestosterone ester. It is used as an injectable and acts as a prodrug of androstanolone.

Boldenone undecylenate, or boldenone undecenoate, sold under the brand names Equipoise and Parenabol among others, is an androgen and anabolic steroid (AAS) medication which is used in veterinary medicine, mainly in horses. It was formerly used in humans as well. It is given by injection into muscle.

Clostebol acetate (BAN), also known as 4-chlorotestosterone 17β-acetate (4-CLTA) or as 4-chloroandrost-4-en-17β-ol-3-one 17β-acetate, is a synthetic, injected anabolic-androgenic steroid (AAS) and a derivative of testosterone that is marketed in Germany and Italy. It is an androgen ester – specifically, the C17β acetate ester of clostebol (4-chlorotestosterone) – and acts as a prodrug of clostebol in the body. Clostebol acetate is administered via intramuscular injection.

Clostebol caproate, also known as clostebol hexanoate or chlorotestosterone caproate (JAN), as well as 4-chlorotestosterone 17β-caproate or as 4-chloroandrost-4-en-17β-ol-3-one 17β-caproate, is a synthetic, injected anabolic-androgenic steroid (AAS) and a derivative of testosterone. It is an androgen ester – specifically, the C17β caproate ester of clostebol (4-chlorotestosterone) – and acts as a prodrug of clostebol in the body. Clostebol acetate is administered via intramuscular injection.
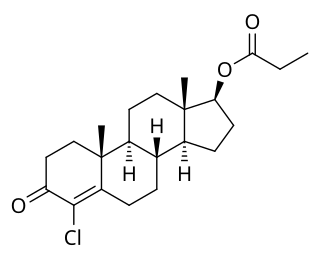
Clostebol propionate, also known as 4-chlorotestosterone 17β-propionate or as 4-chloroandrost-4-en-17β-ol-3-one 17β-propionate, is a synthetic, injected anabolic-androgenic steroid (AAS) and a derivative of testosterone. It is an androgen ester – specifically, the C17β propionate ester of clostebol (4-chlorotestosterone) – and acts as a prodrug of clostebol in the body. Clostebol acetate is administered via intramuscular injection.

Buciclic acid, or bucyclic acid, systematic name trans-4-butylcyclohexane-1-carboxylic acid, is a simple alkyl-substituted cyclohexanecarboxylic acid. The salts and esters of buciclic acid are known as buciclates (bucyclates). Pharmaceutical examples of esters of this acid include testosterone buciclate, a long-acting prodrug of the androgen testosterone, and dimethandrolone buciclate, a prodrug of dimethandrolone.