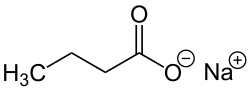
Butyric acid, also known under the systematic name butanoic acid, is a straight-chain alkyl carboxylic acid with the chemical formula CH3CH2CH2CO2H. It is an oily, colorless liquid with an unpleasant odor. Isobutyric acid is an isomer. Salts and esters of butyric acid are known as butyrates or butanoates. The acid does not occur widely in nature, but its esters are widespread. It is a common industrial chemical and an important component in the mammalian gut.

Histone deacetylases (EC 3.5.1.98, HDAC) are a class of enzymes that remove acetyl groups (O=C-CH3) from an ε-N-acetyl lysine amino acid on both histone and non-histone proteins. HDACs allow histones to wrap the DNA more tightly. This is important because DNA is wrapped around histones, and DNA expression is regulated by acetylation and de-acetylation. HDAC's action is opposite to that of histone acetyltransferase. HDAC proteins are now also called lysine deacetylases (KDAC), to describe their function rather than their target, which also includes non-histone proteins. In general, they suppress gene expression.

Trichostatin A (TSA) is an organic compound that serves as an antifungal antibiotic and selectively inhibits the class I and II mammalian histone deacetylase (HDAC) families of enzymes, but not class III HDACs. However, there are recent reports of the interactions of this molecule with Sirt 6 protein. TSA inhibits the eukaryotic cell cycle during the beginning of the growth stage. TSA can be used to alter gene expression by interfering with the removal of acetyl groups from histones and therefore altering the ability of DNA transcription factors to access the DNA molecules inside chromatin. It is a member of a larger class of histone deacetylase inhibitors that have a broad spectrum of epigenetic activities. Thus, TSA has some potential as an anti-cancer drug. One suggested mechanism is that TSA promotes the expression of apoptosis-related genes, leading to cancerous cells surviving at lower rates, thus slowing the progression of cancer. Other mechanisms may include the activity of HDIs to induce cell differentiation, thus acting to "mature" some of the de-differentiated cells found in tumors. HDIs have multiple effects on non-histone effector molecules, so the anti-cancer mechanisms are truly not understood at this time.
A tetrapeptide is a peptide, classified as an oligopeptide, since it only consists of four amino acids joined by peptide bonds. Many tetrapeptides are pharmacologically active, often showing affinity and specificity for a variety of receptors in protein-protein signaling. Present in nature are both linear and cyclic tetrapeptides (CTPs), the latter of which mimics protein reverse turns which are often present on the surface of proteins and druggable targets. Tetrapeptides may be cyclized by a fourth peptide bond or other covalent bonds.
Vorinostat (rINN), also known as suberoylanilide hydroxamic acid, is a member of a larger class of compounds that inhibit histone deacetylases (HDAC). Histone deacetylase inhibitors (HDI) have a broad spectrum of epigenetic activities.

Histone acetylation and deacetylation are the processes by which the lysine residues within the N-terminal tail protruding from the histone core of the nucleosome are acetylated and deacetylated as part of gene regulation.
Histone deacetylase inhibitors are chemical compounds that inhibit histone deacetylases.

Histone deacetylase 2 (HDAC2) is an enzyme that in humans is encoded by the HDAC2 gene. It belongs to the histone deacetylase class of enzymes responsible for the removal of acetyl groups from lysine residues at the N-terminal region of the core histones. As such, it plays an important role in gene expression by facilitating the formation of transcription repressor complexes and for this reason is often considered an important target for cancer therapy.

Histone deacetylase 6 is an enzyme that in humans is encoded by the HDAC6 gene. HDAC6 has emerged as a highly promising candidate to selectively inhibit as a therapeutic strategy to combat several types of cancer and neurodegenerative disorders.

Free fatty acid receptor 2 (FFAR2), also termed G-protein coupled receptor 43 (GPR43), is a rhodopsin-like G-protein coupled receptor. It is coded by the FFAR2 gene. In humans, the FFAR2 gene is located on the long arm of chromosome 19 at position 13.12. Like other GPCRs, FFAR2s reside on the surface membrane of cells and when bond to one of their activating ligands regulate the function of their parent cells. FFAR2 is a member of a small family of structurally and functionally related GPRs termed free fatty acid receptors (FFARs). This family includes three other receptors which, like FFAR2, are activated by certain fatty acids: FFAR1, FFAR3 (GPR41), and FFAR4 (GPR120). FFAR2 and FFAR3 are activated by short-chain fatty acids whereas FFAR1 and FFAR4 are activated by long-chain fatty acids.

Hydroxycarboxylic acid receptor 2 (HCA2), also known as GPR109A and niacin receptor 1 (NIACR1), is a protein which in humans is encoded (its formation is directed) by the HCAR2 gene and in rodents by the Hcar2 gene. The human HCAR2 gene is located on the long (i.e., "q") arm of chromosome 12 at position 24.31 (notated as 12q24.31). Like the two other hydroxycarboxylic acid receptors, HCA1 and HCA3, HCA2 is a G protein-coupled receptor (GPCR) located on the surface membrane of cells. HCA2 binds and thereby is activated by D-β-hydroxybutyric acid (hereafter termed β-hydroxybutyric acid), butyric acid, and niacin (also known as nicotinic acid). β-Hydroxybutyric and butyric acids are regarded as the endogenous agents that activate HCA2. Under normal conditions, niacin's blood levels are too low to do so: it is given as a drug in high doses in order to reach levels that activate HCA2.

Histone deacetylase 5 is an enzyme that in humans is encoded by the HDAC5 gene.

Protein fosB, also known as FosB and G0/G1 switch regulatory protein 3 (G0S3), is a protein that in humans is encoded by the FBJ murine osteosarcoma viral oncogene homolog B (FOSB) gene.
Histone deacetylase 8 is an enzyme that in humans is encoded by the HDAC8 gene.

Romidepsin, sold under the brand name Istodax, is an anticancer agent used in cutaneous T-cell lymphoma (CTCL) and other peripheral T-cell lymphomas (PTCLs). Romidepsin is a natural product obtained from the bacterium Chromobacterium violaceum, and works by blocking enzymes known as histone deacetylases, thus inducing apoptosis. It is sometimes referred to as depsipeptide, after the class of molecules to which it belongs. Romidepsin is branded and owned by Gloucester Pharmaceuticals, a part of Celgene.
Embryonic stem cells are capable of self-renewing and differentiating to the desired fate depending on their position in the body. Stem cell homeostasis is maintained through epigenetic mechanisms that are highly dynamic in regulating the chromatin structure as well as specific gene transcription programs. Epigenetics has been used to refer to changes in gene expression, which are heritable through modifications not affecting the DNA sequence.
Epigenetic regulation of neurogenesis is the role that epigenetics plays in the regulation of neurogenesis.
Epigenetic therapy is the use of drugs or other epigenome-influencing techniques to treat medical conditions. Many diseases, including cancer, heart disease, diabetes, and mental illnesses are influenced by epigenetic mechanisms. Epigenetic therapy offers a potential way to influence those pathways directly.

Neurodegenerative diseases are a heterogeneous group of complex disorders linked by the degeneration of neurons in either the peripheral nervous system or the central nervous system. Their underlying causes are extremely variable and complicated by various genetic and/or environmental factors. These diseases cause progressive deterioration of the neuron resulting in decreased signal transduction and in some cases even neuronal death. Peripheral nervous system diseases may be further categorized by the type of nerve cell affected by the disorder. Effective treatment of these diseases is often prevented by lack of understanding of the underlying molecular and genetic pathology. Epigenetic therapy is being investigated as a method of correcting the expression levels of misregulated genes in neurodegenerative diseases.
Pharmacoepigenetics is an emerging field that studies the underlying epigenetic marking patterns that lead to variation in an individual's response to medical treatment.