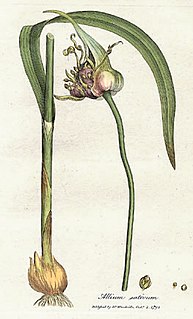
Allicin is an organosulfur compound obtained from garlic, a species in the family Alliaceae. It was first isolated and studied in the laboratory by Chester J. Cavallito and John Hays Bailey in 1944. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. The allicin generated is unstable and quickly changes into a series of other sulfur-containing compounds such as diallyl disulfide. Allicin is part of a defense mechanism against attacks by pests on the garlic plant.

An allyl group is a substituent with the structural formula H2C=CH−CH2R, where R is the rest of the molecule. It consists of a methylene bridge (−CH2−) attached to a vinyl group (−CH=CH2). The name is derived from the Latin word for garlic, Allium sativum. In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "Schwefelallyl". The term allyl applies to many compounds related to H2C=CH−CH2, some of which are of practical or of everyday importance, for example, allyl chloride.

An aroma compound, also known as an odorant, aroma, fragrance, or flavor, is a chemical compound that has a smell or odor. A chemical compound has a smell or odor when it is sufficiently volatile to be transported to the olfactory system in the upper part of the nose.
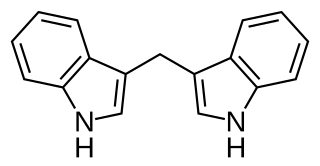
3,3′-Diindolylmethane (DIM) is a compound derived from the digestion of indole-3-carbinol, found in cruciferous vegetables such as broccoli, Brussels sprouts, cabbage and kale. The reputation of Brassica vegetables as healthy foods rests in part on the activities of diindolylmethane.

Allyl isothiocyanate (AITC) is the organosulfur compound with the formula CH2CHCH2NCS. This colorless oil is responsible for the pungent taste of mustard, radish, horseradish, and wasabi. This pungency and the lachrymatory effect of AITC are mediated through the TRPA1 and TRPV1 ion channels. It is slightly soluble in water, but more soluble in most organic solvents.
Ajoene is an organosulfur compound found in garlic extracts. It is a colorless liquid that contains sulfoxide and disulfide functional groups. The name is derived from "ajo", the Spanish word for garlic. It is found as a mixture of up to four stereoisomers, which differ in terms of the stereochemistry of the central alkene and the chirality of the sulfoxide sulfur.
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

A thial or thioaldehyde is a functional group in organic chemistry which is similar to an aldehyde, RC(O)H, in which a sulfur (S) atom replaces the oxygen (O) atom of the aldehyde (R represents an alkyl or aryl group). Thioaldehydes are even more reactive than thioketones. Unhindered thioaldehydes are generally too reactive to be isolated — for example, thioformaldehyde, H2C=S, condenses to the cyclic trimer 1,3,5-trithiane. Thioacrolein, H2C=CHCH=S, formed by decomposition of allicin from garlic, undergoes a self Diels-Alder reaction giving isomeric vinyldithiins. While thioformaldehyde is highly reactive, it is found in interstellar space along with its mono- and di-deuterated isotopologues. With sufficient steric bulk, however, stable thioaldehydes can be isolated.

Diallyl disulfide is an organosulfur compound derived from garlic and a few other genus Allium plants. Along with diallyl trisulfide and diallyl tetrasulfide, it is one of the principal components of the distilled oil of garlic. It is a yellowish liquid which is insoluble in water and has a strong garlic odor. It is produced during the decomposition of allicin, which is released upon crushing garlic and other plants of the Alliaceae family. Diallyl disulfide has many of the health benefits of garlic, but it is also an allergen causing garlic allergy. Highly diluted, it is used as a flavoring in food. It decomposes in the human body into other compounds such as allyl methyl sulfide.

S-Allyl cysteine (SAC) is an organic compound that is a natural constituent of fresh garlic. It is a derivative of the amino acid cysteine in which an allyl group has been added to the sulfur atom.

A sulfenic acid is an organosulfur compound and oxoacid with the general formula RSOH. It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids and sulfonic acids, RSO2H and RSO3H, respectively. The base member of the sulfenic acid series with R = H is hydrogen thioperoxide.

syn-Propanethial S-oxide (C3H6OS), a member of a class of organosulfur compounds known as thiocarbonyl S-oxides (formerly "sulfines"), is a liquid that acts as a lachrymatory agent (triggers tearing and stinging on contact with the eyes). The chemical is released from onions, Allium cepa, as they are sliced. The release is due to the breaking open of the onion cells and their releasing enzymes called alliinases, which then break down amino acid sulfoxides, generating sulfenic acids. A specific sulfenic acid, 1-propenesulfenic acid, formed when onions are cut, is rapidly rearranged by a second enzyme, called the lachrymatory factor synthase or LFS, giving syn-propanethial S-oxide. The gas diffuses through the air and, on contact with the eye, it stimulates sensory neurons creating a stinging, painful sensation. Tears are released from the tear glands to dilute and flush out the irritant. Recently, a structurally related lachrymatory compound, syn-butanethial S-oxide, C4H8OS, has been found in another genus Allium plant, Allium siculum.
1,3-Propane sultone is the organosulfur compound with the formula (CH2)3SO3. It is a cyclic sulfonate ester, a class of compounds called sultones. It is a readily melting colorless solid.
Allyl methyl sulfide (AMS) is an organosulfur compound with the chemical formula CH2=CHCH2SCH3. The molecule features two functional groups, an allyl (CH2=CHCH2) and a sulfide. It is a colourless liquid with a strong odor characteristic of alkyl sulfides. It is a metabolite of garlic, and "garlic breath" is attributed to its presence.
Allyl propyl disulfide is an organosulfur compound with the chemical formula C3H5S2C3H7. It is a volatile pale-yellow liquid with a strong odor. It is a major component of onion oil and is used in food additives and flavors. This substance is present in garlic and onion. When onion or garlic is sliced, the substance evaporates and causes eyes to irritate. When garlic or onion is cooked, it also evaporates, ridding them of the spicy taste, and leaving a sweet taste in them.
Vinyldithiins, more precisely named 3-vinyl-4H-1,2-dithiin and 2-vinyl-4H-1,3-dithiin, are organosulfur phytochemicals formed in the breakdown of allicin from crushed garlic (Allium sativum). Vinyldithiins are Diels-Alder dimers of thioacrolein, H2C=CHCH=S, formed in turn by decomposition of allicin. In garlic supplements, vinyldithiins are only found in garlic oil macerates that are made by incubation of crushed garlic in oil.

Garlic breath is halitosis resulting from the consumption of garlic.
Antifeedants are organic compounds produced by plants to inhibit attack by insects and grazing animals. These chemical compounds are typically classified as secondary metabolites in that they are not essential for the metabolism of the plant, but instead confer longevity. Antifeedants exhibit a wide range of activities and chemical structures as biopesticides. Examples include rosin, which inhibits attack on trees, and many alkaloids, which are highly toxic to specific insect species.

Eric Block is an American chemist whose research has focused on the chemistry of organosulfur and organoselenium compounds, Allium chemistry, and the chemistry of olfaction. As of 2018, he is Distinguished Professor of Chemistry Emeritus at the University at Albany, SUNY.