| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Pyrrolidine-2,5-dione [1] | |||
| Other names Succinimide Succinic acid imide | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.215 | ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties [2] | |||
| C4H5NO2 | |||
| Molar mass | 99.089 g·mol−1 | ||
| Appearance | White crystalline powder | ||
| Density | 1.41 g/cm3 | ||
| Melting point | 125 to 127 °C (257 to 261 °F; 398 to 400 K) | ||
| Boiling point | 287 to 289 °C (549 to 552 °F; 560 to 562 K) | ||
| 0.33 g/mL | |||
| Acidity (pKa) | 9.5 | ||
| −47.3·10−6 cm3/mol | |||
| Pharmacology | |||
| G04BX10 ( WHO ) | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | Irritant Slightly Flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) | 14 g/kg (rat, oral) [2] | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related Imides | Maleimide, N-Chlorosuccinimide, N-Bromosuccinimide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
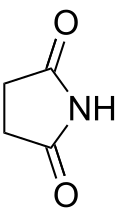
Succinimide is an organic compound with the formula (CH2)2(CO)2NH. This white solid is used in a variety of organic syntheses, as well as in some industrial silver plating processes. The compound is classified as a cyclic imide. It may be prepared by thermal decomposition of ammonium succinate. [3]