
Benzodiazepines, colloquially called "benzos", are a class of depressant drugs whose core chemical structure is the fusion of a benzene ring and a diazepine ring. They are prescribed to treat conditions such as anxiety disorders, insomnia, and seizures. The first benzodiazepine, chlordiazepoxide (Librium), was discovered accidentally by Leo Sternbach in 1955, and was made available in 1960 by Hoffmann–La Roche, which followed with the development of diazepam (Valium) three years later, in 1963. By 1977, benzodiazepines were the most prescribed medications globally; the introduction of selective serotonin reuptake inhibitors (SSRIs), among other factors, decreased rates of prescription, but they remain frequently used worldwide.
Anticonvulsants are a diverse group of pharmacological agents used in the treatment of epileptic seizures. Anticonvulsants are also increasingly being used in the treatment of bipolar disorder and borderline personality disorder, since many seem to act as mood stabilizers, and for the treatment of neuropathic pain. Anticonvulsants suppress the excessive rapid firing of neurons during seizures. Anticonvulsants also prevent the spread of the seizure within the brain.

Diazepam, sold under the brand name Valium among others, is a medicine of the benzodiazepine family that acts as an anxiolytic. It is used to treat a range of conditions, including anxiety, seizures, alcohol withdrawal syndrome, muscle spasms, insomnia, and restless legs syndrome. It may also be used to cause memory loss during certain medical procedures. It can be taken orally, as a suppository inserted into the rectum, intramuscularly, intravenously or used as a nasal spray. When injected intravenously, effects begin in one to five minutes and last up to an hour. When taken by mouth, effects begin after 15 to 60 minutes.
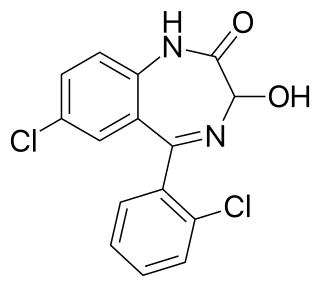
Lorazepam, sold under the brand name Ativan among others, is a benzodiazepine medication. It is used to treat anxiety, trouble sleeping, severe agitation, active seizures including status epilepticus, alcohol withdrawal, and chemotherapy-induced nausea and vomiting. It is also used during surgery to interfere with memory formation and to sedate those who are being mechanically ventilated. It is also used, along with other treatments, for acute coronary syndrome due to cocaine use. It can be given orally, transdermally, intravenously (IV), or intramuscularly When given by injection, onset of effects is between one and thirty minutes and effects last for up to a day.
Depressants, colloquially known as "downers" or central nervous system (CNS) depressants, are drugs that lower neurotransmission levels, decrease the electrical activity of brain cells, or reduce arousal or stimulation in various areas of the brain. Some specific depressants do influence mood, either positively or negatively, but depressants often have no clear impact on mood. In contrast, stimulants, or "uppers", increase mental alertness, making stimulants the opposite drug class from depressants. Antidepressants are defined by their effect on mood, not on general brain activity, so they form an orthogonal category of drugs.

Clonazepam, sold under the brand name Klonopin among others, is a medication used to prevent and treat anxiety disorders, seizures, bipolar mania, agitation associated with psychosis, obsessive–compulsive disorder, and akathisia. It is a long-acting tranquilizer of the benzodiazepine class. It possesses anxiolytic, anticonvulsant, sedative, hypnotic, and skeletal muscle relaxant properties. It is typically taken orally but is also used intravenously. Effects begin within one hour and last between eight and twelve hours in adults.

The GABAA receptor (GABAAR) is an ionotropic receptor and ligand-gated ion channel. Its endogenous ligand is γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Accurate regulation of GABAergic transmission through appropriate developmental processes, specificity to neural cell types, and responsiveness to activity is crucial for the proper functioning of nearly all aspects of the central nervous system (CNS). Upon opening, the GABAA receptor on the postsynaptic cell is selectively permeable to chloride ions and, to a lesser extent, bicarbonate ions.

Phenobarbital, also known as phenobarbitone or phenobarb, sold under the brand name Luminal among others, is a medication of the barbiturate type. It is recommended by the World Health Organization (WHO) for the treatment of certain types of epilepsy in developing countries. In the developed world, it is commonly used to treat seizures in young children, while other medications are generally used in older children and adults. It is also used for veterinary purposes. It may be administered by slow intravenous infusion, intramuscularly (IM), or orally. Subcutaneous administration is not recommended. The IV or IM may be used to treat status epilepticus if other drugs fail to achieve satisfactory results. Phenobarbital is occasionally used to treat insomnia, anxiety, and benzodiazepine withdrawal, and prior to surgery as an anxiolytic and to induce sedation. It usually begins working within five minutes when used intravenously and half an hour when administered orally. Its effects last for between four hours and two days.

Status epilepticus (SE), or status seizure, is a medical condition consisting of a single seizure lasting more than 5 minutes, or 2 or more seizures within a 5-minute period without the person returning to normal between them. Previous definitions used a 30-minute time limit. The seizures can be of the tonic–clonic type, with a regular pattern of contraction and extension of the arms and legs, or of types that do not involve contractions, such as absence seizures or complex partial seizures. Status epilepticus is a life-threatening medical emergency, particularly if treatment is delayed.
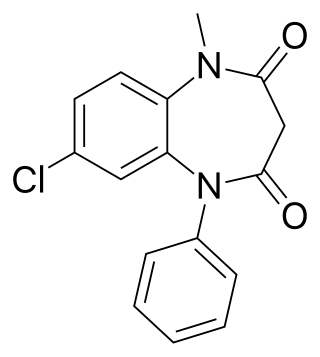
Clobazam, sold under the brand names Frisium, Onfi and others, is a benzodiazepine class medication that was patented in 1968. Clobazam was first synthesized in 1966 and first published in 1969. Clobazam was originally marketed as an anxioselective anxiolytic since 1970, and an anticonvulsant since 1984. The primary drug-development goal was to provide greater anxiolytic, anti-obsessive efficacy with fewer benzodiazepine-related side effects.

Clorazepate, sold under the brand name Tranxene among others, is a benzodiazepine medication. It possesses anxiolytic, anticonvulsant, sedative, hypnotic, and skeletal muscle relaxant properties. Clorazepate is an unusually long-lasting benzodiazepine and serves as a prodrug for the equally long-lasting desmethyldiazepam, which is rapidly produced as an active metabolite. Desmethyldiazepam is responsible for most of the therapeutic effects of clorazepate.

Alpidem, sold under the brand name Ananxyl, is a nonbenzodiazepine anxiolytic medication which was briefly used to treat anxiety disorders but is no longer marketed. It was previously marketed in France, but was discontinued due to liver toxicity. Alpidem is taken by mouth.

Bretazenil (Ro16-6028) is an imidazopyrrolobenzodiazepine anxiolytic drug which is derived from the benzodiazepine family, and was invented in 1988. It is most closely related in structure to the GABA antagonist flumazenil, although its effects are somewhat different. It is classified as a high-potency benzodiazepine due to its high affinity binding to benzodiazepine binding sites where it acts as a partial agonist. Its profile as a partial agonist and preclinical trial data suggests that it may have a reduced adverse effect profile. In particular bretazenil has been proposed to cause a less strong development of tolerance and withdrawal syndrome. Bretazenil differs from traditional 1,4-benzodiazepines by being a partial agonist and because it binds to α1, α2, α3, α4, α5 and α6 subunit containing GABAA receptor benzodiazepine receptor complexes. 1,4-benzodiazepines bind only to α1, α2, α3 and α5GABAA benzodiazepine receptor complexes.

Imidazenil is an experimental anxiolytic drug which is derived from the benzodiazepine family, and is most closely related to other imidazobenzodiazepines such as midazolam, flumazenil, and bretazenil.

Abecarnil (ZK-112,119) is an anxiolytic drug from the β-Carboline family. It is one of a relatively recently developed class of medicines known as the nonbenzodiazepines, which have similar effects to the older benzodiazepine group, but with quite different chemical structures. It is a partial agonist acting selectively at the benzodiazepine site of the GABAA receptor.

Pipequaline (INN) is an anxiolytic drug that was never marketed. It possesses a novel chemical structure that is not closely related to other drugs of this type. The drug has a similar pharmacological profile to the benzodiazepine family of drugs, but with mainly anxiolytic properties and very little sedative, amnestic or anticonvulsant effects, and so is classified as a nonbenzodiazepine anxiolytic.

Loreclezole is a sedative and an anticonvulsant which acts as a GABAA receptor positive allosteric modulator. The binding site of loreclezole has been shown experimentally to be shared by valerenic acid, an extract of the root of the valerian plant. Structurally, loreclezole is a triazole derivative. In animal seizure models, loreclezole is protective against pentylenetetrazol seizures but is less active in the maximal electroshock test. In addition, at low, nontoxic doses, the drug has anti-absence activity in a genetic model of generalized absence epilepsy. Consequently, loreclezole has a profile of activity similar to that of benzodiazepines. A potential benzodiazepine-like interaction with GABA receptors is suggested by the observation that the anticonvulsant effects of loreclezole can be reversed by benzodiazepine receptor inverse agonists. The benzodiazepine antagonist flumazenil, however, fails to alter the anticonvulsant activity of loreclezole, indicating that loreclezole is not a benzodiazepine receptor agonist. Using native rat and cloned human GABA-A receptors, loreclezole strongly potentiated GABA-activated chloride current. However, the activity of the drug did not require the presence of the γ-subunit and was not blocked by flumazenil, confirming that loreclezole does not interact with the benzodiazepine recognition site.

ELB-139 (LS-191,811) is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.
A convulsant is a drug which induces convulsions and/or epileptic seizures, the opposite of an anticonvulsant. These drugs generally act as stimulants at low doses, but are not used for this purpose due to the risk of convulsions and consequent excitotoxicity. Most convulsants are antagonists at either the GABAA or glycine receptors, or ionotropic glutamate receptor agonists. Many other drugs may cause convulsions as a side effect at high doses but only drugs whose primary action is to cause convulsions are known as convulsants. Nerve agents such as sarin, which were developed as chemical weapons, produce convulsions as a major part of their toxidrome, but also produce a number of other effects in the body and are usually classified separately. Dieldrin which was developed as an insecticide blocks chloride influx into the neurons causing hyperexcitability of the CNS and convulsions. The Irwin observation test and other studies that record clinical signs are used to test the potential for a drug to induce convulsions. Camphor, and other terpenes given to children with colds can act as convulsants in children who have had febrile seizures.
















