
Hypnotic, or soporific drugs, commonly known as sleeping pills, are a class of psychoactive drugs whose primary function is to induce sleep and to treat insomnia (sleeplessness).

Sevoflurane, sold under the brand name Sevorane, among others, is a sweet-smelling, nonflammable, highly fluorinated methyl isopropyl ether used as an inhalational anaesthetic for induction and maintenance of general anesthesia. After desflurane, it is the volatile anesthetic with the fastest onset. While its offset may be faster than agents other than desflurane in a few circumstances, its offset is more often similar to that of the much older agent isoflurane. While sevoflurane is only half as soluble as isoflurane in blood, the tissue blood partition coefficients of isoflurane and sevoflurane are quite similar. For example, in the muscle group: isoflurane 2.62 vs. sevoflurane 2.57. In the fat group: isoflurane 52 vs. sevoflurane 50. As a result, the longer the case, the more similar will be the emergence times for sevoflurane and isoflurane.

General anaesthesia (UK) or general anesthesia (US) is a method of medically inducing loss of consciousness that renders a patient unarousable even with painful stimuli. This effect is achieved by administering either intravenous or inhalational general anaesthetic medications, which often act in combination with an analgesic and neuromuscular blocking agent. Spontaneous ventilation is often inadequate during the procedure and intervention is often necessary to protect the airway. General anaesthesia is generally performed in an operating theater to allow surgical procedures that would otherwise be intolerably painful for a patient, or in an intensive care unit or emergency department to facilitate endotracheal intubation and mechanical ventilation in critically ill patients. Depending on the procedure, general anaesthesia may be optional or required. Regardless of whether a patient may prefer to be unconscious or not, certain pain stimuli could result in involuntary responses from the patient that may make an operation extremely difficult. Thus, for many procedures, general anaesthesia is required from a practical perspective.
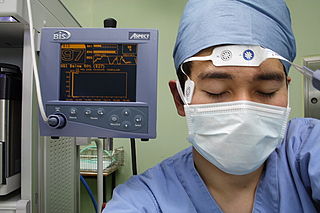
Bispectral index (BIS) is one of several technologies used to monitor depth of anesthesia. BIS monitors are used to supplement Guedel's classification system for determining depth of anesthesia. Titrating anesthetic agents to a specific bispectral index during general anesthesia in adults allows the anesthetist to adjust the amount of anesthetic agent to the needs of the patient, possibly resulting in a more rapid emergence from anesthesia. Use of the BIS monitor could reduce the incidence of intraoperative awareness during anaesthesia. The exact details of the algorithm used to create the BIS index have not been disclosed by the company that developed it.
Premedication is using medication before some other therapy to prepare for that forthcoming therapy. Typical examples include premedicating with a sedative or analgesic before surgery; using prophylactic (preventive) antibiotics before surgery; and using antiemetics or antihistamines before chemotherapy.

Remifentanil, marketed under the brand name Ultiva is a potent, short-acting synthetic opioid analgesic drug. It is given to patients during surgery to relieve pain and as an adjunct to an anaesthetic. Remifentanil is used for sedation as well as combined with other medications for use in general anesthesia. The use of remifentanil has made possible the use of high-dose opioid and low-dose hypnotic anesthesia, due to synergism between remifentanil and various hypnotic drugs and volatile anesthetics.

Zaleplon, sold under the brand name Sonata among others, is a sedative and hypnotic which is used to treat insomnia. It is a nonbenzodiazepine or Z-drug of the pyrazolopyrimidine class. It was developed by King Pharmaceuticals and approved for medical use in the United States in 1999.

Nonbenzodiazepines, sometimes referred to colloquially as Z-drugs, are a class of psychoactive, depressant, sedative, hypnotic, anxiolytic drugs that are benzodiazepine-like in uses, such as for treating insomnia and anxiety.

Quazepam, sold under brand name Doral among others, is a relatively long-acting benzodiazepine derivative drug developed by the Schering Corporation in the 1970s. Quazepam is used for the treatment of insomnia including sleep induction and sleep maintenance. Quazepam induces impairment of motor function and has relatively selective hypnotic and anticonvulsant properties with considerably less overdose potential than other benzodiazepines. Quazepam is an effective hypnotic which induces and maintains sleep without disruption of the sleep architecture.
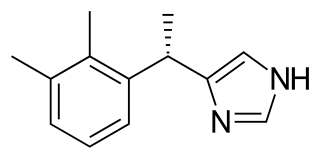
Dexmedetomidine, sold under the trade name Precedex among others, is a drug used in humans for sedation. Veterinarians use dexmedetomidine for similar purposes in treating cats, dogs, and horses. It is also used in humans to treat acute agitation associated with schizophrenia or bipolar I or II disorder.

Alfaxalone, also known as alphaxalone or alphaxolone and sold under the brand name Alfaxan, is a neuroactive steroid and general anesthetic which is used currently in veterinary practice as an induction agent for anesthesia and as an injectable anesthetic. Though it is more expensive than other induction agents, it often preferred due to the lack of depressive effects on the cardiovascular system. The most common side effect seen in current veterinary practice is respiratory depression when Alfaxan is administered concurrently with other sedative and anesthetic drugs; when premedications aren't given, veterinary patients also become agitated and hypersensitive when waking up.

Rilmazafone is a water-soluble prodrug developed in Japan. Inside the human body, rilmazafone is converted into several benzodiazepine metabolites that have sedative and hypnotic effects.

CL-218,872 is a sedative and hypnotic drug used in scientific research. It has similar effects to sedative-hypnotic benzodiazepine drugs such as triazolam, but is structurally distinct and so is classed as a nonbenzodiazepine hypnotic.

Y-23684 is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.

SX-3228 is a sedative and hypnotic drug used in scientific research. It has similar effects to sedative-hypnotic benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine hypnotic.

Ro48-6791 is a drug, an imidazobenzodiazepine derivative developed by Hoffman-LaRoche in the 1990s.

Pentamorphone is a semi-synthetic opiate derivative related to compounds such as Morphinone and oxymorphone. Developed in 1984, it is a potent opioid analgesic several times stronger than fentanyl, and with a similarly fast onset of effects and short duration of action. It was found to produce relatively little respiratory depression compared to other potent opioid agonists, but its analgesic effects were somewhat disappointing in human trials, and while pentamorphone had some slight advantages over fentanyl these were not sufficient to warrant its introduction into clinical use.

Frakefamide (INN) is a synthetic, fluorinated linear tetrapeptide with the amino acid sequence Tyr-D-Ala-(p-F)Phe-Phe-NH2 which acts as a peripherally-specific, selective μ-opioid receptor agonist. Despite its inability to penetrate the blood-brain-barrier and enter the central nervous system, frakefamide has potent analgesic effects and, unlike centrally-acting opioids like morphine, does not produce respiratory depression, indicating that its antinociceptive effects are mediated by peripheral μ-opioid receptors. It was under development for the treatment of pain by AstraZeneca and Shire but was shelved after phase II clinical trials.

In pharmacology, GABAA receptor positive allosteric modulators, also known as GABAkines or GABAA receptor potentiators, are positive allosteric modulator (PAM) molecules that increase the activity of the GABAA receptor protein in the vertebrate central nervous system.
Total intravenous anesthesia (TIVA) refers to the intravenous administration of anesthetic agents to induce a temporary loss of sensation or awareness. The first study of TIVA was done in 1872 using chloral hydrate, and the common anesthetic agent propofol was licensed in 1986. TIVA is currently employed in various procedures as an alternative technique of general anesthesia in order to improve post-operative recovery.


















