Related Research Articles
Free-running sleep is a rare sleep pattern whereby the sleep schedule of a person shifts later every day. It occurs as the sleep disorder non-24-hour sleep–wake disorder or artificially as part of experiments used in the study of circadian and other rhythms in biology. Study subjects are shielded from all time cues, often by a constant light protocol, by a constant dark protocol or by the use of light/dark conditions to which the organism cannot entrain such as the ultrashort protocol of one hour dark and two hours light. Also, limited amounts of food may be made available at short intervals so as to avoid entrainment to mealtimes. Subjects are thus forced to live by their internal circadian "clocks".
Jet lag is a physiological condition that results from alterations to the body's circadian rhythms caused by rapid long-distance trans-meridian travel. For example, someone flying from New York to London, i.e. from west to east, feels as if the time were five hours earlier than local time, and someone travelling from London to New York, i.e. from east to west, feels as if the time were five hours later than local time. The phase shift when traveling from east to west is referred to as phase-delay of the circadian circle, whereas going west to east is phase-advance of the circadian circle. Most travelers find that it is harder to timezone adjust when traveling to the east. Jet lag was previously classified as one of the circadian rhythm sleep disorders.

A circadian rhythm, or circadian cycle, is a natural, internal process that regulates the sleep–wake cycle and repeats roughly every 24 hours. It can refer to any process that originates within an organism and responds to the environment. These 24-hour rhythms are driven by a circadian clock, and they have been widely observed in animals, plants, fungi and cyanobacteria.

Chronobiology is a field of biology that examines timing processes, including periodic (cyclic) phenomena in living organisms, such as their adaptation to solar- and lunar-related rhythms. These cycles are known as biological rhythms. Chronobiology comes from the ancient Greek χρόνος, and biology, which pertains to the study, or science, of life. The related terms chronomics and chronome have been used in some cases to describe either the molecular mechanisms involved in chronobiological phenomena or the more quantitative aspects of chronobiology, particularly where comparison of cycles between organisms is required.
The pineal gland, conarium, or epiphysis cerebri, is a small endocrine gland in the brain of most vertebrates. The pineal gland produces melatonin, a serotonin-derived hormone which modulates sleep patterns in both circadian and seasonal cycles. The shape of the gland resembles a pine cone, which gives it its name. The pineal gland is located in the epithalamus, near the center of the brain, between the two hemispheres, tucked in a groove where the two halves of the thalamus join. The pineal gland is one of the neuroendocrine secretory circumventricular organs in which capillaries are mostly permeable to solutes in the blood.

Melatonin is a natural product found in plants and animals. It is primarily known in animals as a hormone released by the pineal gland in the brain at night, and has long been associated with control of the sleep–wake cycle.

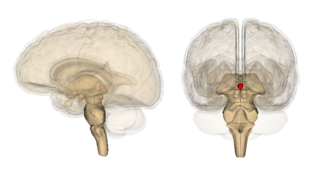
The suprachiasmatic nucleus or nuclei (SCN) is a tiny region of the brain in the hypothalamus, situated directly above the optic chiasm. It is responsible for controlling circadian rhythms. The neuronal and hormonal activities it generates regulate many different body functions in a 24-hour cycle. The mouse SCN contains approximately 20,000 neurons.

Pinealocytes are the main cells contained in the pineal gland, located behind the third ventricle and between the two hemispheres of the brain. The primary function of the pinealocytes is the secretion of the hormone melatonin, important in the regulation of circadian rhythms. In humans, the suprachiasmatic nucleus of the hypothalamus communicates the message of darkness to the pinealocytes, and as a result, controls the day and night cycle. It has been suggested that pinealocytes are derived from photoreceptor cells. Research has also shown the decline in the number of pinealocytes by way of apoptosis as the age of the organism increases. There are two different types of pinealocytes, type I and type II, which have been classified based on certain properties including shape, presence or absence of infolding of the nuclear envelope, and composition of the cytoplasm.

Melanopsin is a type of photopigment belonging to a larger family of light-sensitive retinal proteins called opsins and encoded by the gene Opn4. In the mammalian retina, there are two additional categories of opsins, both involved in the formation of visual images: rhodopsin and photopsin in the rod and cone photoreceptor cells, respectively.
Non-24-hour sleep–wake disorder is one of several chronic circadian rhythm sleep disorders (CRSDs). It is defined as a "chronic steady pattern comprising [...] daily delays in sleep onset and wake times in an individual living in a society". Symptoms result when the non-entrained (free-running) endogenous circadian rhythm drifts out of alignment with the light–dark cycle in nature. Although this sleep disorder is more common in blind people, affecting up to 70% of the totally blind, it can also affect sighted people. Non-24 may also be comorbid with bipolar disorder, depression, and traumatic brain injury. The American Academy of Sleep Medicine (AASM) has provided CRSD guidelines since 2007 with the latest update released in 2015.
Intrinsically photosensitive retinal ganglion cells (ipRGCs), also called photosensitive retinal ganglion cells (pRGC), or melanopsin-containing retinal ganglion cells (mRGCs), are a type of neuron in the retina of the mammalian eye. The presence of ipRGCs was first suspected in 1927 when rodless, coneless mice still responded to a light stimulus through pupil constriction, This implied that rods and cones are not the only light-sensitive neurons in the retina. Yet research on these cells did not advance until the 1980s. Recent research has shown that these retinal ganglion cells, unlike other retinal ganglion cells, are intrinsically photosensitive due to the presence of melanopsin, a light-sensitive protein. Therefore they constitute a third class of photoreceptors, in addition to rod and cone cells.
Circadian rhythm sleep disorders (CRSD), also known as circadian rhythm sleep-wake disorders (CRSWD), are a family of sleep disorders which affect the timing of sleep. CRSDs arise from a persistent pattern of sleep/wake disturbances that can be caused either by dysfunction in one's biological clock system, or by misalignment between one's endogenous oscillator and externally imposed cues. As a result of this mismatch, those affected by circadian rhythm sleep disorders have a tendency to fall asleep at unconventional time points in the day. These occurrences often lead to recurring instances of disturbed rest, where individuals affected by the disorder are unable to go to sleep and awaken at "normal" times for work, school, and other social obligations. Delayed sleep phase disorder, advanced sleep phase disorder, non-24-hour sleep–wake disorder and irregular sleep–wake rhythm disorder represents the four main types of CRSD.
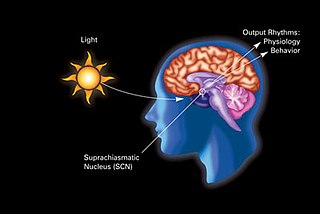
In neuroanatomy, the retinohypothalamic tract (RHT) is a photic neural input pathway involved in the circadian rhythms of mammals. The origin of the retinohypothalamic tract is the intrinsically photosensitive retinal ganglion cells (ipRGC), which contain the photopigment melanopsin. The axons of the ipRGCs belonging to the retinohypothalamic tract project directly, monosynaptically, to the suprachiasmatic nuclei (SCN) via the optic nerve and the optic chiasm. The suprachiasmatic nuclei receive and interpret information on environmental light, dark and day length, important in the entrainment of the "body clock". They can coordinate peripheral "clocks" and direct the pineal gland to secrete the hormone melatonin.

Melatonin receptor type 1A is a protein that in humans is encoded by the MTNR1A gene.
Light effects on circadian rhythm are the effects that light has on circadian rhythm.

Melatonin receptor 1B, also known as MTNR1B, is a protein that in humans is encoded by the MTNR1B gene.
Steven M. Reppert is an American neuroscientist known for his contributions to the fields of chronobiology and neuroethology. His research has focused primarily on the physiological, cellular, and molecular basis of circadian rhythms in mammals and more recently on the navigational mechanisms of migratory monarch butterflies. He was the Higgins Family Professor of Neuroscience at the University of Massachusetts Medical School from 2001 to 2017, and from 2001 to 2013 was the founding chair of the Department of Neurobiology. Reppert stepped down as chair in 2014. He is currently distinguished professor emeritus of neurobiology.

Melatonin receptor agonists are analogues of melatonin that bind to and activate the melatonin receptor. Agonists of the melatonin receptor have a number of therapeutic applications including treatment of sleep disorders and depression. The discovery and development of melatonin receptor agonists was motivated by the need for more potent analogues than melatonin, with better pharmacokinetics and longer half-lives. Melatonin receptor agonists were developed with the melatonin structure as a model.
A chronobiotic is an agent that can cause phase adjustment of the circadian rhythm. That is, it is a substance capable of therapeutically entraining or re-entraining long-term desynchronized or short-term dissociated circadian rhythms in mammals, or prophylactically preventing their disruption following an environmental insult such as is caused by rapid travel across several time zones. The most widely recognized chronobiotic is the hormone melatonin, secreted at night in both diurnal and nocturnal species.
Michael Menaker, was an American chronobiology researcher, and was Commonwealth Professor of Biology at University of Virginia. His research focused on circadian rhythmicity of vertebrates, including contributing to an understanding of light input pathways on extra-retinal photoreceptors of non-mammalian vertebrates, discovering a mammalian mutation for circadian rhythmicity, and locating a circadian oscillator in the pineal gland of bird. He wrote almost 200 scientific publications.
References
- ↑ Reppert SM (December 1997). "Melatonin receptors: molecular biology of a new family of G protein-coupled receptors". Journal of Biological Rhythms. 12 (6): 528–531. doi:10.1177/074873049701200606. PMID 9406026. S2CID 6501856.
- ↑ Reppert SM, Weaver DR, Godson C (March 1996). "Melatonin receptors step into the light: cloning and classification of subtypes". Trends in Pharmacological Sciences. 17 (3): 100–102. doi:10.1016/0165-6147(96)10005-5. PMID 8936344.
- 1 2 Sugden D, Davidson K, Hough KA, Teh MT (October 2004). "Melatonin, melatonin receptors and melanophores: a moving story". Pigment Cell Research. 17 (5): 454–460. doi: 10.1111/j.1600-0749.2004.00185.x . PMID 15357831.
- 1 2 Doghramji K (August 2007). "Melatonin and its receptors: a new class of sleep-promoting agents". Journal of Clinical Sleep Medicine. 3 (5 Suppl): S17–S23. doi:10.5664/jcsm.26932. PMC 1978320 . PMID 17824497.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Emet M, Ozcan H, Ozel L, Yayla M, Halici Z, Hacimuftuoglu A (June 2016). "A Review of Melatonin, Its Receptors and Drugs". The Eurasian Journal of Medicine. 48 (2): 135–141. doi:10.5152/eurasianjmed.2015.0267. PMC 4970552 . PMID 27551178.
- ↑ Stauch B, Johansson LC, McCorvy JD, Patel N, Han GW, Huang XP, et al. (May 2019). "Structural basis of ligand recognition at the human MT1 melatonin receptor". Nature. 569 (7755): 284–288. Bibcode:2019Natur.569..284S. doi:10.1038/s41586-019-1141-3. PMC 6696938 . PMID 31019306.
- ↑ Johansson LC, Stauch B, McCorvy JD, Han GW, Patel N, Huang XP, et al. (May 2019). "XFEL structures of the human MT2 melatonin receptor reveal the basis of subtype selectivity". Nature. 569 (7755): 289–292. Bibcode:2019Natur.569..289J. doi:10.1038/s41586-019-1144-0. PMC 6589158 . PMID 31019305.
- ↑ Stauch B, Johansson LC, Cherezov V (April 2020). "Structural insights into melatonin receptors". The FEBS Journal. 287 (8): 1496–1510. doi: 10.1111/febs.15128 . PMC 7174090 . PMID 31693784.
- ↑ Okamoto HH, Miyauchi H, Inoue A, Raimondi F, Tsujimoto H, Kusakizako T, et al. (August 2021). "Cryo-EM structure of the human MT1-Gi signaling complex". Nature Structural & Molecular Biology. 28 (8): 694–701. doi:10.1038/s41594-021-00634-1. PMID 34354246. S2CID 236935241.
- 1 2 Zlotos DP, Jockers R, Cecon E, Rivara S, Witt-Enderby PA (April 2014). "MT1 and MT2 melatonin receptors: ligands, models, oligomers, and therapeutic potential". Journal of Medicinal Chemistry. 57 (8): 3161–3185. doi:10.1021/jm401343c. PMID 24228714.
- 1 2 3 4 5 6 7 8 9 Dubocovich ML (December 2007). "Melatonin receptors: role on sleep and circadian rhythm regulation". Sleep Medicine. 8 (Suppl 3): 34–42. doi:10.1016/j.sleep.2007.10.007. PMID 18032103.
- ↑ Lacoste B, Angeloni D, Dominguez-Lopez S, Calderoni S, Mauro A, Fraschini F, et al. (May 2015). "Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain". Journal of Pineal Research. 58 (4): 397–417. doi:10.1111/jpi.12224. hdl: 11382/504527 . PMID 25726952. S2CID 42979051.
- ↑ Morgan PJ, Barrett P, Howell HE, Helliwell R (February 1994). "Melatonin receptors: localization, molecular pharmacology and physiological significance". Neurochemistry International. 24 (2): 101–146. doi:10.1016/0197-0186(94)90100-7. PMID 8161940. S2CID 26865785.
- 1 2 Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF (September 1995). "Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor". Proceedings of the National Academy of Sciences of the United States of America. 92 (19): 8734–8738. Bibcode:1995PNAS...92.8734R. doi: 10.1073/pnas.92.19.8734 . PMC 41041 . PMID 7568007.
- ↑ Besharse JC, Dunis DA (March 1983). "Methoxyindoles and photoreceptor metabolism: activation of rod shedding". Science. 219 (4590): 1341–1343. Bibcode:1983Sci...219.1341B. doi:10.1126/science.6828862. PMID 6828862.
- ↑ Ochoa-Sanchez R, Comai S, Lacoste B, Bambico FR, Dominguez-Lopez S, Spadoni G, et al. (December 2011). "Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand". The Journal of Neuroscience. 31 (50): 18439–18452. doi:10.1523/JNEUROSCI.2676-11.2011. PMC 6623882 . PMID 22171046.
- ↑ Ochoa-Sanchez R, Rainer Q, Comai S, Spadoni G, Bedini A, Rivara S, et al. (December 2012). "Anxiolytic effects of the melatonin MT(2) receptor partial agonist UCM765: comparison with melatonin and diazepam". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 39 (2): 318–325. doi:10.1016/j.pnpbp.2012.07.003. PMID 22789661. S2CID 40097067.
- ↑ US8791163B2,Gobbi F, Mor M, Rivara S, Fraschini F,"Melatonin ligands having antidepressant activity as well as sleep inducing properties",issued 2014-07-29
- ↑ Pévet P (October 2016). "Melatonin receptors as therapeutic targets in the suprachiasmatic nucleus". Expert Opinion on Therapeutic Targets. 20 (10): 1209–1218. doi:10.1080/14728222.2016.1179284. PMID 27082492. S2CID 25868288.