Secretin is a hormone that regulates water homeostasis throughout the body and influences the environment of the duodenum by regulating secretions in the stomach, pancreas, and liver. It is a peptide hormone produced in the S cells of the duodenum, which are located in the intestinal glands. In humans, the secretin peptide is encoded by the SCT gene.
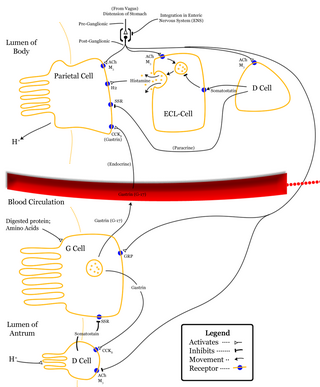
Delta cells are somatostatin-producing cells. They can be found in the stomach, intestine and the pancreatic islets. Delta cells comprise ca 5% of the cells in the islets but may interact with many more islet cells than suggested by their low numbers. In rodents, delta-cells are located in the periphery of the islets; in humans the islet architecture is generally less organized and delta-cells are frequently observed inside the islets as well. In both species, the peptide hormone Urocortin III (Ucn3) is a major local signal that is released from beta cells to induce the local secretion of somatostatin. It has also been suggested that somatostatin may be implicated in insulin-induced hypoglycaemia through a mechanism involving SGLT-2 receptors. Ghrelin can also strongly stimulate somatostatin secretion, thus indirectly inhibiting insulin release. Viewed under an electron microscope, delta-cells can be identified as cells with smaller and slightly more compact granules than beta cells.

Vasoactive intestinal peptide, also known as vasoactive intestinal polypeptide or VIP, is a peptide hormone that is vasoactive in the intestine. VIP is a peptide of 28 amino acid residues that belongs to a glucagon/secretin superfamily, the ligand of class II G protein–coupled receptors. VIP is produced in many tissues of vertebrates including the gut, pancreas, cortex, and suprachiasmatic nuclei of the hypothalamus in the brain. VIP stimulates contractility in the heart, causes vasodilation, increases glycogenolysis, lowers arterial blood pressure and relaxes the smooth muscle of trachea, stomach and gallbladder. In humans, the vasoactive intestinal peptide is encoded by the VIP gene.

Guanylate cyclase 2C, also known as guanylyl cyclase C (GC-C), intestinal guanylate cyclase, guanylate cyclase-C receptor, or the heat-stable enterotoxin receptor (hSTAR) is an enzyme that in humans is encoded by the GUCY2C gene.
There are two known receptors for the vasoactive intestinal peptide (VIP) termed VPAC1 and VPAC2. These receptors bind both VIP and pituitary adenylate cyclase-activating polypeptide (PACAP) to some degree. Both receptors are members of the 7 transmembrane G protein-coupled receptor family.

Pituitary adenylate cyclase-activating polypeptide also known as PACAP is a protein that in humans is encoded by the ADCYAP1 gene. pituitary adenylate cyclase-activating polypeptide is similar to vasoactive intestinal peptide. One of its effects is to stimulate enterochromaffin-like cells. It binds to vasoactive intestinal peptide receptor and to the pituitary adenylate cyclase-activating polypeptide receptor.

The secretin receptor is a protein that in humans is encoded by the SCTR gene. This protein is a G protein-coupled receptor which binds secretin and is the leading member of the secretin receptor family, also called class B GPCR subfamily.

Peptide PHI, also known as peptide histidine isoleucine, is a peptide which functions as a hormone. This peptide contains a composition of 27 amino acids with histidine on the N-terminus and isoleucine on the C-terminus. It was originally isolated from the mammalian small intestine amongst mammalian neurons called intramural neurons which function in the motor activity of the intestinal walls. An example of this was revealed in a study that demonstrated that this peptide regulates water and electrolyte transportation in the human jejunum; similar to its inhibitory effects on fluid absorption in the small intestine of pigs and rats.

The gastric inhibitory polypeptide receptor (GIP-R), also known as the glucose-dependent insulinotropic polypeptide receptor, is a protein that in humans is encoded by the GIPR gene.

Pituitary adenylate cyclase-activating polypeptide type I receptor also known as PAC1, is a protein that in humans is encoded by the ADCYAP1R1 gene. This receptor binds pituitary adenylate cyclase activating peptide.

Radiation-inducible immediate-early gene IEX-1 is a protein that in humans is encoded by the IER3 gene.

Corticotropin-releasing hormone receptor 2 (CRHR2) is a protein, also known by the IUPHAR-recommended name CRF2, that is encoded by the CRHR2 gene and occurs on the surfaces of some mammalian cells. CRF2 receptors are type 2 G protein-coupled receptors for corticotropin-releasing hormone (CRH) that are resident in the plasma membranes of hormone-sensitive cells. CRH, a peptide of 41 amino acids synthesized in the hypothalamus, is the principal neuroregulator of the hypothalamic-pituitary-adrenal axis, signaling via guanine nucleotide-binding proteins (G proteins) and downstream effectors such as adenylate cyclase. The CRF2 receptor is a multi-pass membrane protein with a transmembrane domain composed of seven helices arranged in a V-shape. CRF2 receptors are activated by two structurally similar peptides, urocortin II, and urocortin III, as well as CRH.

G-protein coupled receptor 3 is a protein that in humans is encoded by the GPR3 gene. The protein encoded by this gene is a member of the G protein-coupled receptor family of transmembrane receptors and is involved in signal transduction.
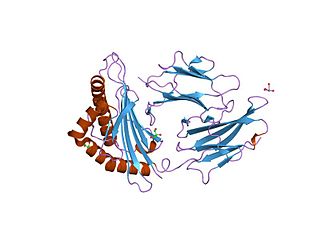
Vasoactive intestinal polypeptide receptor 1 also known as VPAC1, is a protein, that in humans is encoded by the VIPR1 gene. VPAC1 is expressed in the brain (cerebral cortex, hippocampus, amygdala), lung, prostate, peripheral blood leukocytes, liver, small intestine, heart, spleen, placenta, kidney, thymus and testis.
Secretin receptor family consists of secretin receptors regulated by peptide hormones from the glucagon hormone family. The family is different from adhesion G protein-coupled receptors.

Adenylyl cyclase type 6 is an enzyme that in humans is encoded by the ADCY6 gene.

Adenylyl cyclase type 9 is an enzyme that in humans is encoded by the ADCY9 gene.

Adenylyl cyclase type 4 is an enzyme that in humans is encoded by the ADCY4 gene.
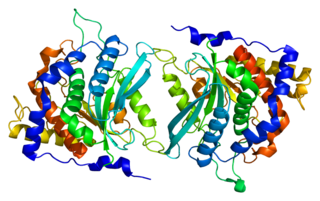
Glutaminyl-peptide cyclotransferase is an enzyme that in humans is encoded by the QPCT gene.
Elizabeth Maywood is an English researcher who studies circadian rhythms and sleep in mice. Her studies are focused on the suprachiasmatic nucleus (SCN), a small region of the brain that controls circadian rhythms.