
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times. Ligands can bind either to extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists although a spontaneous auto-activation of an empty receptor can also be observed.

Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibers in the parasympathetic nervous system.

Endothelins are peptides with receptors and effects in many body organs. Endothelin constricts blood vessels and raises blood pressure. The endothelins are normally kept in balance by other mechanisms, but when overexpressed, they contribute to high blood pressure (hypertension), heart disease, and potentially other diseases.
Calcitonin gene-related peptide (CGRP) is a member of the calcitonin family of peptides consisting of calcitonin, amylin, adrenomedullin, adrenomedullin 2 (intermedin) and calcitonin‑receptor‑stimulating peptide. Calcitonin is mainly produced by thyroid C cells whilst CGRP is secreted and stored in the nervous system. This peptide, in humans, exists in two forms: CGRP alpha, and CGRP beta. α-CGRP is a 37-amino acid neuropeptide and is formed by alternative splicing of the calcitonin/CGRP gene located on chromosome 11. β-CGRP is less studied. In humans, β-CGRP differs from α-CGRP by three amino acids and is encoded in a separate, nearby gene. The CGRP family includes calcitonin (CT), adrenomedullin (AM), and amylin (AMY).

Kisspeptins are proteins encoded by the KISS1 gene in humans. Kisspeptins are ligands of the G-protein coupled receptor, GPR54. Kiss1 was originally identified as a human metastasis suppressor gene that has the ability to suppress melanoma and breast cancer metastasis. Kisspeptin-GPR54 signaling has an important role in initiating secretion of gonadotropin-releasing hormone (GnRH) at puberty, the extent of which is an area of ongoing research. Gonadotropin-releasing hormone is released from the hypothalamus to act on the anterior pituitary triggering the release of luteinizing hormone (LH), and follicle stimulating hormone (FSH). These gonadotropic hormones lead to sexual maturation and gametogenesis. Disrupting GPR54 signaling can cause hypogonadotrophic hypogonadism in rodents and humans. The Kiss1 gene is located on chromosome 1. It is transcribed in the brain, adrenal gland, and pancreas.

The glucagon-like peptide-1 receptor (GLP1R) is a receptor protein found on beta cells of the pancreas and on neurons of the brain. It is involved in the control of blood sugar level by enhancing insulin secretion. In humans it is synthesised by the gene GLP1R, which is present on chromosome 6. It is a member of the glucagon receptor family of G protein-coupled receptors. GLP1R is composed of two domains, one extracellular (ECD) that binds the C-terminal helix of GLP-1, and one transmembrane (TMD) domain that binds the N-terminal region of GLP-1. In the TMD domain there is a fulcrum of polar residues that regulates the biased signaling of the receptor while the transmembrane helical boundaries and extracellular surface are a trigger for biased agonism.
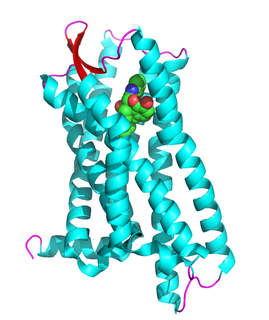
The δ-opioid receptor, also known as delta opioid receptor or simply delta receptor, abbreviated DOR or DOP, is an inhibitory 7-transmembrane G-protein coupled receptor coupled to the G protein Gi/G0 and has enkephalins as its endogenous ligands. The regions of the brain where the δ-opioid receptor is largely expressed vary from species model to species model. In humans, the δ-opioid receptor is most heavily expressed in the basal ganglia and neocortical regions of the brain.

Urotensin-II (U-II) is a peptide ligand that is the strongest known vasoconstrictor. Because of the involvement of the UII system in multiple biological systems such as the cardiovascular, nervous, endocrine, and renal, it represents a promising target for the development of new drugs.

The KiSS1-derived peptide receptor is a G protein-coupled receptor which binds the peptide hormone kisspeptin (metastin). Kisspeptin is encoded by the metastasis suppressor gene KISS1, which is expressed in a variety of endocrine and gonadal tissues. Activation of the kisspeptin receptor is linked to the phospholipase C and inositol trisphosphate second messenger cascades inside the cell.

The muscarinic acetylcholine receptor, also known as cholinergic/acetylcholine receptor M3, or the muscarinic 3, is a muscarinic acetylcholine receptor encoded by the human gene CHRM3.

G protein-coupled receptor 35 also known as GPR35 is a G protein-coupled receptor which in humans is encoded by the GPR35 gene. Heightened expression of GPR35 is found in immune and gastrointestinal tissues, including the crypts of Lieberkühn.

Uracil nucleotide/cysteinyl leukotriene receptor is a G protein-coupled receptor that in humans is encoded by the GPR17 gene located on chromosome 2 at position q21. The actual activating ligands for and some functions of this receptor are disputed.

Hydroxycarboxylic acid receptor 3 (HCA3), also known as niacin receptor 2 (NIACR2) and GPR109B, is a protein which in humans is encoded by the HCAR3 gene. HCA3, like the other hydroxycarboxylic acid receptors HCA1 and HCA2, is a Gi/o-coupled G protein-coupled receptor (GPCR). The primary endogenous agonists of HCA3 are 3-hydroxyoctanoic acid and kynurenic acid. HCA3 is also a low-affinity biomolecular target for niacin (aka nicotinic acid).

Prostaglandin D2 receptor 2 (DP2 or CRTH2) is a human protein encoded by the PTGDR2 gene and GPR44. DP2 has also been designated as CD294 (cluster of differentiation 294). It is a member of the class of prostaglandin receptors which bind with and respond to various prostaglandins. DP2 along with Prostaglandin DP1 receptor are receptors for prostaglandin D2 (PGD2). Activation of DP2 by PGD2 or other cognate receptor ligands has been associated with certain physiological and pathological responses, particularly those associated with allergy and inflammation, in animal models and certain human diseases.

G protein-coupled receptor 119 also known as GPR119 is a G protein-coupled receptor that in humans is encoded by the GPR119 gene.

Hydroxycarboxylic acid receptor 2 (HCA2), also known as niacin receptor 1 (NIACR1) and GPR109A, is a protein which in humans is encoded by the HCAR2 gene. HCA2, like the other hydroxycarboxylic acid receptors HCA1 and HCA3, is a Gi/o-coupled G protein-coupled receptor (GPCR). The primary endogenous agonists of HCA2 are D-β-hydroxybutyric acid and butyric acid (and their conjugate bases, β-hydroxybutyrate and butyrate). HCA2 is also a high-affinity biomolecular target for niacin (aka nicotinic acid).

G-protein coupled receptor 3 is a protein that in humans is encoded by the GPR3 gene. The protein encoded by this gene is a member of the G protein-coupled receptor family of transmembrane receptors and is involved in signal transduction.

G protein-coupled receptor kinase 5 is a member of the G protein-coupled receptor kinase subfamily of the Ser/Thr protein kinases, and is most highly similar to GRK4 and GRK6. The protein phosphorylates the activated forms of G protein-coupled receptors to regulate their signaling.

Formyl peptide receptor 1 is a cell surface receptor protein that in humans is encoded by the formyl peptide receptor 1 (FPR1) gene. This gene encodes a G protein-coupled receptor cell surface protein that binds and is activated by N-Formylmethionine-containing oligopeptides, particularly N-Formylmethionine-leucyl-phenylalanine (FMLP). FPR1 is prominently expressed by mammalian phagocytic and blood leukocyte cells where it functions to mediate these cells' responses to the N-formylmethionine-containing oligopeptides which are released by invading microorganisms and injured tissues. FPR1 directs these cells to sites of invading pathogens or disrupted tissues and then stimulates these cells to kill the pathogens or to remove tissue debris; as such, it is an important component of the innate immune system that operates in host defense and damage control.

Urotensin II-related peptide (URP) is a cyclic neuropeptide that is found in all vertebrates that have been genome sequenced so far. It has a long lasting hypotensive effect and may also regulate reproduction. It is part of the Urotensin II system and is one of the two endogenous ligands for rats, mice, and possibly humans.


















