The classical complement pathway is one of three pathways which activate the complement system, which is part of the immune system. The classical complement pathway is initiated by antigen-antibody complexes with the antibody isotypes IgG and IgM.

Anaphylatoxins, or complement peptides, are fragments that are produced as part of the activation of the complement system. Complement components C3, C4 and C5 are large glycoproteins that have important functions in the immune response and host defense. They have a wide variety of biological activities and are proteolytically activated by cleavage at a specific site, forming a- and b-fragments. A-fragments form distinct structural domains of approximately 76 amino acids, coded for by a single exon within the complement protein gene. The C3a, C4a and C5a components are referred to as anaphylatoxins: they cause smooth muscle contraction, vasodilation, histamine release from mast cells, and enhanced vascular permeability. They also mediate chemotaxis, inflammation, and generation of cytotoxic oxygen radicals. The proteins are highly hydrophilic, with a mainly alpha-helical structure held together by 3 disulfide bridges.

C5a is a protein fragment released from cleavage of complement component C5 by protease C5-convertase into C5a and C5b fragments. C5b is important in late events of the complement cascade, an orderly series of reactions which coordinates several basic defense mechanisms, including formation of the membrane attack complex (MAC), one of the most basic weapons of the innate immune system, formed as an automatic response to intrusions from foreign particles and microbial invaders. It essentially pokes microscopic pinholes in these foreign objects, causing loss of water and sometimes death. C5a, the other cleavage product of C5, acts as a highly inflammatory peptide, encouraging complement activation, formation of the MAC, attraction of innate immune cells, and histamine release involved in allergic responses. The origin of C5 is in the hepatocyte, but its synthesis can also be found in macrophages, where it may cause local increase of C5a. C5a is a chemotactic agent and an anaphylatoxin; it is essential in the innate immunity but it is also linked with the adaptive immunity. The increased production of C5a is connected with a number of inflammatory diseases.

Complement component 5 is a protein that in humans is encoded by the C5 gene.

Mannan-binding lectin serine protease 1 also known as mannose-associated serine protease 1 (MASP-1) is an enzyme that in humans is encoded by the MASP1 gene.

Complement receptor type 2 (CR2), also known as complement C3d receptor, Epstein-Barr virus receptor, and CD21, is a protein that in humans is encoded by the CR2 gene.
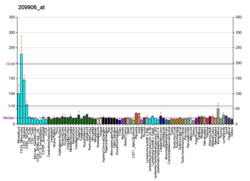
C-X-C motif chemokine 5 is a protein that in humans is encoded by the CXCL5 gene.
Complement component 4 (C4), in humans, is a protein involved in the intricate complement system, originating from the human leukocyte antigen (HLA) system. It serves a number of critical functions in immunity, tolerance, and autoimmunity with the other numerous components. Furthermore, it is a crucial factor in connecting the recognition pathways of the overall system instigated by antibody-antigen (Ab-Ag) complexes to the other effector proteins of the innate immune response. For example, the severity of a dysfunctional complement system can lead to fatal diseases and infections. Complex variations of it can also lead to schizophrenia. The C4 protein was thought to derive from a simple two-locus allelic model, which however has been replaced by a much more sophisticated multimodular RCCX gene complex model which contain long and short forms of the C4A or C4B genes usually in tandem RCCX cassettes with copy number variation, that somewhat parallels variation in the levels of their respective proteins within a population along with CYP21 in some cases depending on the number of cassettes and whether it contains the functional gene instead of pseudogenes or fragments. Originally defined in the context of the Chido/Rodgers blood group system, the C4A-C4B genetic model is under investigation for its possible role in schizophrenia risk and development.

The C5a receptor also known as complement component 5a receptor 1 (C5AR1) or CD88 is a G protein-coupled receptor for C5a. It functions as a complement receptor. C5a receptor 1 modulates inflammatory responses, obesity, development and cancers. From a signaling transduction perspective, C5a receptor 1 activation is implicated in β-arrestin2 recruitment via Rab5a, coupling of Gαi proteins, ERK1/2 phosphorylation, calcium mobilization and Rho activation leading to downstream functions, such as secretion of cytokines, chemotaxis, and phagocytosis.

N-formyl peptide receptor 2 (FPR2) is a G-protein coupled receptor (GPCR) located on the surface of many cell types of various animal species. The human receptor protein is encoded by the FPR2 gene and is activated to regulate cell function by binding any one of a wide variety of ligands including not only certain N-Formylmethionine-containing oligopeptides such as N-Formylmethionine-leucyl-phenylalanine (FMLP) but also the polyunsaturated fatty acid metabolite of arachidonic acid, lipoxin A4 (LXA4). Because of its interaction with lipoxin A4, FPR2 is also commonly named the ALX/FPR2 or just ALX receptor.

N-formyl peptide receptor 3 (FPR3) is a receptor protein that in humans is encoded by the FPR3 gene.

P2Y purinoceptor 14 is a protein that in humans is encoded by the P2RY14 gene.

C5a anaphylatoxin chemotactic receptor 2 is a protein that in humans is encoded by the C5AR2 gene. It's a complement component G protein-coupled receptor, of class A (rhodopsin-like).

CD93 is a protein that in humans is encoded by the CD93 gene. CD93 is a C-type lectin transmembrane receptor which plays a role not only in cell–cell adhesion processes but also in host defense.

C-type lectin domain family 7 member A or Dectin-1 is a protein that in humans is encoded by the CLEC7A gene. CLEC7A is a member of the C-type lectin/C-type lectin-like domain (CTL/CTLD) superfamily. The encoded glycoprotein is a small type II membrane receptor with an extracellular C-type lectin-like domain fold and a cytoplasmic domain with a partial immunoreceptor tyrosine-based activation motif. It functions as a pattern-recognition receptor for a variety of β-1,3-linked and β-1,6-linked glucans from fungi and plants, and in this way plays a role in innate immune response. Expression is found on myeloid dendritic cells, monocytes, macrophages and B cells. Alternate transcriptional splice variants, encoding different isoforms, have been characterized. This gene is closely linked to other CTL/CTLD superfamily members on chromosome 12p13 in the natural killer gene complex region.

CD84 is a human protein encoded by the CD84 gene.

Complement C4-A is a kind of the Complement component 4 protein that in humans is encoded by the C4A gene.

C3a is one of the proteins formed by the cleavage of complement component 3; the other is C3b. C3a is a 77 residue anaphylatoxin that binds to the C3a receptor (C3aR), a class A G protein-coupled receptor. It plays a large role in the immune response.
Complement 3 (C3) through its interaction with factors B and D (adipsin) generates C3a. In the human body, C3a is rapidly cleaved by carboxypeptidase B or carbxyopeptidase N, that remove the carboxyl-terminal arginine to generate C3adesArg. Thus, most of plasmatic C3a is present in C3adesArg form. C3adesArg is more commonly named ASP or acylation-stimulating-protein due to its marked stimulating action on triacylglycerol synthesis in human adipocytes and skin fibroblasts. ASP is also known for its augmentation of glucose transport and inhibiting action on hormone-sensitive lipase. Because of these actions, it is linked to the pathogenesis of obesity, having been demonstrated to be present at increased levels in patients with obesity, diabetes mellitus type 2 and coronary artery disease.

Formyl peptide receptor 1 is a cell surface receptor protein that in humans is encoded by the formyl peptide receptor 1 (FPR1) gene. This gene encodes a G protein-coupled receptor cell surface protein that binds and is activated by N-Formylmethionine-containing oligopeptides, particularly N-Formylmethionine-leucyl-phenylalanine (FMLP). FPR1 is prominently expressed by mammalian phagocytic and blood leukocyte cells where it functions to mediate these cells' responses to the N-formylmethionine-containing oligopeptides which are released by invading microorganisms and injured tissues. FPR1 directs these cells to sites of invading pathogens or disrupted tissues and then stimulates these cells to kill the pathogens or to remove tissue debris; as such, it is an important component of the innate immune system that operates in host defense and damage control.