G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in form of six loops of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Ligands can bind either to the extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists, although a spontaneous auto-activation of an empty receptor has also been observed.

In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and produce physiological responses such as change in the electrical activity of a cell. For example, GABA, an inhibitory neurotransmitter inhibits electrical activity of neurons by binding to GABAA receptors. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand, and integration allows the signal to be incorporated into another biochemical pathway.
Second messengers are intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers. Second messengers trigger physiological changes at cellular level such as proliferation, differentiation, migration, survival, apoptosis and depolarization.
In biology, cell signaling or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. Cell signaling is a fundamental property of all cellular life in prokaryotes and eukaryotes. Signals that originate from outside a cell can be physical agents like mechanical pressure, voltage, temperature, light, or chemical signals. Cell signaling can occur over short or long distances, and as a result can be classified as autocrine, juxtacrine, intracrine, paracrine, or endocrine. Signaling molecules can be synthesized from various biosynthetic pathways and released through passive or active transports, or even from cell damage.

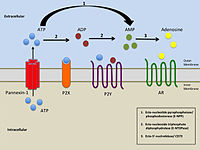
P2Y12 is a chemoreceptor for adenosine diphosphate (ADP) that belongs to the Gi class of a group of G protein-coupled (GPCR) purinergic receptors. This P2Y receptor family has several receptor subtypes with different pharmacological selectivity, which overlaps in some cases, for various adenosine and uridine nucleotides. The P2Y12 receptor is involved in platelet aggregation and is thus a biological target for the treatment of thromboembolisms and other clotting disorders. Two transcript variants encoding the same isoform have been identified for this gene.

Purinergic receptors, also known as purinoceptors, are a family of plasma membrane molecules that are found in almost all mammalian tissues. Within the field of purinergic signalling, these receptors have been implicated in learning and memory, locomotor and feeding behavior, and sleep. More specifically, they are involved in several cellular functions, including proliferation and migration of neural stem cells, vascular reactivity, apoptosis and cytokine secretion. These functions have not been well characterized and the effect of the extracellular microenvironment on their function is also poorly understood.

The P2X receptors, also ATP-gated P2X receptor cation channel family, is a protein family that consists of cation-permeable ligand-gated ion channels that open in response to the binding of extracellular adenosine 5'-triphosphate (ATP). They belong to a larger family of receptors known as the ENaC/P2X superfamily. ENaC and P2X receptors have similar 3-D structures and are homologous. P2X receptors are present in a diverse array of organisms including humans, mouse, rat, rabbit, chicken, zebrafish, bullfrog, fluke, and amoeba.

Ectonucleotidases consist of families of nucleotide metabolizing enzymes that are expressed on the plasma membrane and have externally oriented active sites. These enzymes metabolize nucleotides to nucleosides. The contribution of ectonucleotidases in the modulation of purinergic signaling depends on the availability and preference of substrates and on cell and tissue distribution.

P2Y purinoceptor 1 is a protein that in humans is encoded by the P2RY1 gene.

P2Y purinoceptor 2 is a protein that in humans is encoded by the P2RY2 gene.

Uracil nucleotide/cysteinyl leukotriene receptor is a G protein-coupled receptor that in humans is encoded by the GPR17 gene located on chromosome 2 at position q21. The actual activating ligands for and some functions of this receptor are disputed.

P2Y purinoceptor 6 is a protein that in humans is encoded by the P2RY6 gene.

P2Y purinoceptor 11 is a protein that in humans is encoded by the P2RY11 gene.

P2Y purinoceptor 14 is a protein that in humans is encoded by the P2RY14 gene.

Lysophosphatidic acid receptor 6, also known as LPA6, P2RY5 and GPR87, is a protein that in humans is encoded by the LPAR6 gene. LPA6 is a G protein-coupled receptor that binds the lipid signaling molecule lysophosphatidic acid (LPA).

2-Oxoglutarate receptor 1 (OXGR1), also known as cysteinyl leukotriene receptor E (CysLTE) and GPR99, is a protein that in humans is encoded by the OXGR1 gene. The Gene has recently been nominated as a receptor not only for 2-oxogluterate but also for the three cysteinyl leukotrienes (CysLTs), particularly leukotriene E4 (LTE4) and to far lesser extents LTC4 and LTE4. Recent studies implicate GPR99 as a cellular receptor which is activated by LTE4 thereby causing these cells to contribute to mediating various allergic and hypersensitivity responses.

Putative P2Y purinoceptor 10 is a protein that, in humans, is encoded by the P2RY10 gene.

P2Y purinoceptor 4 is a protein that in humans is encoded by the P2RY4 gene.
P2 receptor may refer to:

Purinergic signalling is a form of extracellular signalling mediated by purine nucleotides and nucleosides such as adenosine and ATP. It involves the activation of purinergic receptors in the cell and/or in nearby cells, thereby regulating cellular functions.