Gap junctions are membrane channels between adjacent cells that allow the direct exchange of cytoplasmic substances. Substances exchanged include small molecules, substrates, and metabolites.

Cell adhesion is the process by which cells interact and attach to neighbouring cells through specialised molecules of the cell surface. This process can occur either through direct contact between cell surfaces such as cell junctions or indirect interaction, where cells attach to surrounding extracellular matrix, a gel-like structure containing molecules released by cells into spaces between them. Cells adhesion occurs from the interactions between cell-adhesion molecules (CAMs), transmembrane proteins located on the cell surface. Cell adhesion links cells in different ways and can be involved in signal transduction for cells to detect and respond to changes in the surroundings. Other cellular processes regulated by cell adhesion include cell migration and tissue development in multicellular organisms. Alterations in cell adhesion can disrupt important cellular processes and lead to a variety of diseases, including cancer and arthritis. Cell adhesion is also essential for infectious organisms, such as bacteria or viruses, to cause diseases.

In biology, a connexon, also known as a connexin hemichannel, is an assembly of six proteins called connexins that form the pore for a gap junction between the cytoplasm of two adjacent cells. This channel allows for bidirectional flow of ions and signaling molecules. The connexon is the hemichannel supplied by a cell on one side of the junction; two connexons from opposing cells normally come together to form the complete intercellular gap junction channel. In some cells, the hemichannel itself is active as a conduit between the cytoplasm and the extracellular space, allowing the transference of ions and small molecules lower than 1-2 KDa. Little is known about this function of connexons besides the new evidence suggesting their key role in intracellular signaling. In still other cells connexons have been shown to occur in mitochondrial membranes and appear to play a role in heart ischaemia.

Connexins (Cx), or gap junction proteins, are structurally related transmembrane proteins that assemble to form vertebrate gap junctions. An entirely different family of proteins, the innexins, forms gap junctions in invertebrates. Each gap junction is composed of two hemichannels, or connexons, which consist of homo- or heterohexameric arrays of connexins, and the connexon in one plasma membrane docks end-to-end with a connexon in the membrane of a closely opposed cell. The hemichannel is made of six connexin subunits, each of which consist of four transmembrane segments. Gap junctions are essential for many physiological processes, such as the coordinated depolarization of cardiac muscle, proper embryonic development, and the conducted response in microvasculature. Connexins also have non-channel dependant functions relating to cytoskeleton and cell migration. For these reasons, mutations in connexin-encoding genes can lead to functional and developmental abnormalities.

Cell junctions or junctional complexes are a class of cellular structures consisting of multiprotein complexes that provide contact or adhesion between neighboring cells or between a cell and the extracellular matrix in animals. They also maintain the paracellular barrier of epithelia and control paracellular transport. Cell junctions are especially abundant in epithelial tissues. Combined with cell adhesion molecules and extracellular matrix, cell junctions help hold animal cells together.
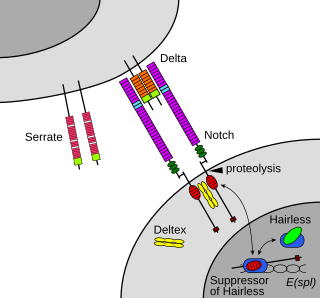
In biology, juxtracrine signalling is a type of cell–cell or cell–extracellular matrix signalling in multicellular organisms that requires close contact. In this type of signalling, a ligand on one surface binds to a receptor on another adjacent surface. Hence, this stands in contrast to releasing a signaling molecule by diffusion into extracellular space, the use of long-range conduits like membrane nanotubes and cytonemes or the use of extracellular vesicles like exosomes or microvesicles. There are three types of juxtracrine signaling:
- A membrane-bound ligand and a membrane protein of two adjacent cells interact.
- A communicating junction links the intracellular compartments of two adjacent cells, allowing transit of relatively small molecules.
- An extracellular matrix glycoprotein and a membrane protein interact.
Innexins are transmembrane proteins that form gap junctions in invertebrates. Gap junctions are composed of membrane proteins that form a channel permeable to ions and small molecules connecting the cytoplasm of adjacent cells. Although gap junctions provide similar functions in all multicellular organisms, it was not known what proteins invertebrates used for this purpose until the late 1990s. While the connexin family of gap junction proteins was well-characterized in vertebrates, no homologues were found in non-chordates.
Membrane channels are a family of biological membrane proteins which allow the passive movement of ions, water (aquaporins) or other solutes to passively pass through the membrane down their electrochemical gradient. They are studied using a range of channelomics experimental and mathematical techniques. Insights have suggested endocannabinoids (eCBs) as molecules that can regulate the opening of these channels during diverse conditions.

Gap junction beta-1 protein (GJB1), also known as connexin 32 (Cx32), is a transmembrane protein that in humans is encoded by the GJB1 gene. Gap junction beta-1 protein is a member of the gap junction connexin family of proteins that regulates and controls the transfer of communication signals across cell membranes, primarily in the liver and peripheral nervous system. However, the protein is expressed in multiple organs, including in oligodendrocytes in the central nervous system.

Gap junction alpha-8 protein is a protein that in humans is encoded by the GJA8 gene. It is also known as connexin 50.
Cell–cell interaction refers to the direct interactions between cell surfaces that play a crucial role in the development and function of multicellular organisms. These interactions allow cells to communicate with each other in response to changes in their microenvironment. This ability to send and receive signals is essential for the survival of the cell. Interactions between cells can be stable such as those made through cell junctions. These junctions are involved in the communication and organization of cells within a particular tissue. Others are transient or temporary such as those between cells of the immune system or the interactions involved in tissue inflammation. These types of intercellular interactions are distinguished from other types such as those between cells and the extracellular matrix. The loss of communication between cells can result in uncontrollable cell growth and cancer.

Hereditary mucoepithelial dysplasia (HMD), or simply mucoepithelial dysplasia, is a rare autosomal dominant multiepithelial disorder causing systemic maldevelopment of the epithelia and mucous membranes that line the surface of tissues and structures throughout the body, particularly affecting systems affiliated with mucosa, which includes the respiratory, digestive, urinary, reproductive and immune systems. The disorder is attributed to improper formation of desmosomes and gap junctions, which prevents proper cornification of the epithelial layer of the skin.

Gap junction delta-2 (GJD2), also known as connexin-36 (Cx36) or gap junction alpha-9 (GJA9), is a protein that in humans is encoded by the GJD2 gene.

Gap junction alpha-10 protein, also known as connexin-62 (Cx62), is a protein that in humans is encoded by the GJA10 gene.

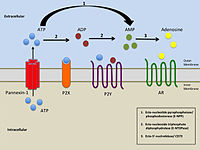
Pannexin 1 is a protein in humans that is encoded by the PANX1 gene.
Anoxic depolarization is a progressive and uncontrollable depolarization of neurons during stroke or brain ischemia in which there is an inadequate supply of blood to the brain. Anoxic depolarization is induced by the loss of neuronal selective membrane permeability and the ion gradients across the membrane that are needed to support neuronal activity. Normally, the Na+/K+-ATPase pump maintains the transmembrane gradients of K+ and Na+ ions, but with anoxic brain injury, the supply of energy to drive this pump is lost. The hallmarks of anoxic depolarization are increased concentrations of extracellular K+ ions, intracellular Na+ and Ca2+ ions, and extracellular glutamate and aspartate. Glutamate and aspartate are normally present as the brain's primary excitatory neurotransmitters, but high concentrations activate a number of downstream apoptotic and necrotic pathways. This results in neuronal dysfunction and brain death.

Gap junction modulation describes the functional manipulation of gap junctions, specialized channels that allow direct electrical and chemical communication between cells without exporting material from the cytoplasm. Gap junctions play an important regulatory role in various physiological processes including signal propagation in cardiac muscles and tissue homeostasis of the liver. Modulation is required, since gap junctions must respond to their environment, whether through an increased expression or permeability. Impaired or altered modulation can have significant health implications and are associated with the pathogenesis of the liver, heart and intestines.
Vinnexin is a transmembrane protein whose DNA code is held in a virus genome. When the virus genome is expressed in a cell the vinnexin gene from the virus is made into a functioning protein by the infected cell. The vinnexin protein is then incorporated into the host's cell membranes to alter the way the hosts cells communicate with each other. The altered communication aids the transmission and replication of the virus in complex ways. The communication structure that the vinnexin is involved in is the gap junction and vinnexin forms part of a wider family of proteins that are innexin homologues referred to as pannexins. So far Vinnexins have only been found in Adenovirus and the way they affect the functioning of innexins is being studied in great detail.
Intercellular communication (ICC) refers to the various ways and structures that biological cells use to communicate with each other directly or through their environment. Often the environment has been thought of as the extracellular spaces within an animal. More broadly cells may also communicate with other animals, either of their own group or species, or other species in the wider ecosystem. Different types of cells use different proteins and mechanisms to communicate with one another using extracellular signalling molecules or electric fluctuations which could be likened to an intercellular ethernet. Components of each type of intercellular communication may be involved in more than one type of communication making attempts at clearly separating the types of communication listed somewhat futile. Broadly speaking, intercellular communication may be categorized as being within a single animal, or between an animal and other animals in the ecosystem in which it lives. In this article intercellular communication has been further collated into various areas of research rather than by functional or structural characteristics.
Gap junction modulators are compounds or agents that either facilitate or inhibit the transfer of small molecules between biological cells by regulating gap junctions. Various physiological processes including cardiac, neural or auditory, depend on gap junctions to perform crucial regulatory roles, and the modulators themselves are the key players in this procedure. Gap junctions are necessary for diffusion of small molecules from cell to cell, keeping the cells interlinked and connecting the cytoplasm, allowing transfer of signals or resources between the body.