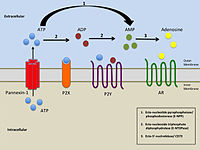
The P2X receptors, also ATP-gated P2X receptor cation channel family, is a protein family that consists of cation-permeable ligand-gated ion channels that open in response to the binding of extracellular adenosine 5'-triphosphate (ATP). They belong to a larger family of receptors known as the ENaC/P2X superfamily. ENaC and P2X receptors have similar 3-D structures and are homologous. P2X receptors are present in a diverse array of organisms including humans, mouse, rat, rabbit, chicken, zebrafish, bullfrog, fluke, and amoeba.

Satellite glial cells, formerly called amphicytes, are glial cells that cover the surface of neuron cell bodies in ganglia of the peripheral nervous system. Thus, they are found in sensory, sympathetic, and parasympathetic ganglia. Both satellite glial cells (SGCs) and Schwann cells are derived from the neural crest of the embryo during development. SGCs have been found to play a variety of roles, including control over the microenvironment of sympathetic ganglia. They are thought to have a similar role to astrocytes in the central nervous system (CNS). They supply nutrients to the surrounding neurons and also have some structural function. Satellite cells also act as protective, cushioning cells. Additionally, they express a variety of receptors that allow for a range of interactions with neuroactive chemicals. Many of these receptors and other ion channels have recently been implicated in health issues including chronic pain and herpes simplex. There is much more to be learned about these cells, and research surrounding additional properties and roles of the SGCs is ongoing.

P2Y receptors are a family of purinergic G protein-coupled receptors, stimulated by nucleotides such as adenosine triphosphate, adenosine diphosphate, uridine triphosphate, uridine diphosphate and UDP-glucose.To date, 8 P2Y receptors have been cloned in humans: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14.

Ectonucleotidases consist of families of nucleotide metabolizing enzymes that are expressed on the plasma membrane and have externally oriented active sites. These enzymes metabolize nucleotides to nucleosides. The contribution of ectonucleotidases in the modulation of purinergic signaling depends on the availability and preference of substrates and on cell and tissue distribution.

P2X purinoceptor 7 is a protein that in humans is encoded by the P2RX7 gene.

P2Y purinoceptor 1 is a protein that in humans is encoded by the P2RY1 gene.

P2X purinoceptor 1, also ATP receptor, is a protein that in humans is encoded by the P2RX1 gene.

P2Y purinoceptor 2 is a protein that in humans is encoded by the P2RY2 gene.

P2Y purinoceptor 11 is a protein that in humans is encoded by the P2RY11 gene.

P2Y purinoceptor 14 is a protein that in humans is encoded by the P2RY14 gene.

Putative P2Y purinoceptor 10 is a protein that, in humans, is encoded by the P2RY10 gene.
P2 receptor may refer to:

P2X purinoceptor 4 is a protein that in humans is encoded by the P2RX4 gene. P2X purinoceptor 4 is a member of the P2X receptor family. P2X receptors are trimeric protein complexes that can be homomeric or heteromeric. These receptors are ligand-gated cation channels that open in response to ATP binding. Each receptor subtype, determined by the subunit composition, varies in its affinity to ATP and desensitization kinetics.

P2X purinoceptor 5 is a protein in humans that is encoded by the P2RX5 gene.
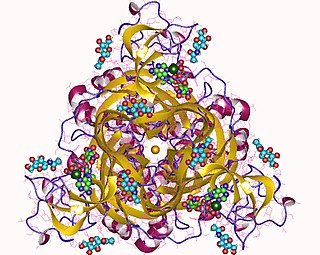
P2X purinoceptor 3 is a protein that in humans is encoded by the P2RX3 gene.

P2X purinoceptor 6 is a protein that in humans is encoded by the P2RX6 gene.
The rostral ventromedial medulla (RVM), or ventromedial nucleus of the spinal cord, is a group of neurons located close to the midline on the floor of the medulla oblongata. The rostral ventromedial medulla sends descending inhibitory and excitatory fibers to the dorsal horn spinal cord neurons. There are 3 categories of neurons in the RVM: on-cells, off-cells, and neutral cells. They are characterized by their response to nociceptive input. Off-cells show a transitory decrease in firing rate right before a nociceptive reflex, and are theorized to be inhibitory. Activation of off-cells, either by morphine or by any other means, results in antinociception. On-cells show a burst of activity immediately preceding nociceptive input, and are theorized to be contributing to the excitatory drive. Neutral cells show no response to nociceptive input.

PPADS (pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid) is a selective purinergic P2X antagonist. It is able to block contractions of rabbit vas deferens induced by ATP or α,β,methylene-ATP. It appears to be relatively selective for P2X receptors, having no appreciable activity at α1 adrenergic, muscarinic M2 and M3, histamine H1, and adenosine A1 receptors.

Purinergic signalling is a form of extracellular signalling mediated by purine nucleotides and nucleosides such as adenosine and ATP. It involves the activation of purinergic receptors in the cell and/or in nearby cells, thereby regulating cellular functions.
Microglia are the primary immune cells of the central nervous system, similar to peripheral macrophages. They respond to pathogens and injury by changing morphology and migrating to the site of infection/injury, where they destroy pathogens and remove damaged cells.