Contents
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 1,3,7,9-Tetramethyl-7,9-dihydro-1H-purine-2,6,8(3H)-trione | |
| Other names 1,3,7,9-Tetramethyluric acid; Temurin; Temorine; Tetramethyluric acid; Tetramethyl uric acid; TeaCrine (trade name) | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.017.268 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C9H12N4O3 | |
| Molar mass | 224.220 g·mol−1 |
| Melting point | 226 °C (439 °F; 499 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
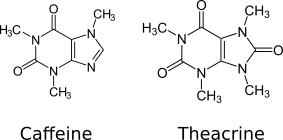
Theacrine, also known as 1,3,7,9-tetramethyluric acid, is a purine alkaloid found in cupuaçu (Theobroma grandiflorum) and in a Chinese kucha tea (Chinese : 苦 茶 ; pinyin :kǔ chá; lit.'bitter tea') ( Camellia assamica var. kucha). [1] [2] It shows anti-inflammatory and analgesic effects and appears to affect adenosine signalling in a manner similar to caffeine. [2] [3] In kucha leaves, theacrine is synthesized from caffeine in what is thought to be a three-step pathway. [2] Theacrine and caffeine are structurally similar.