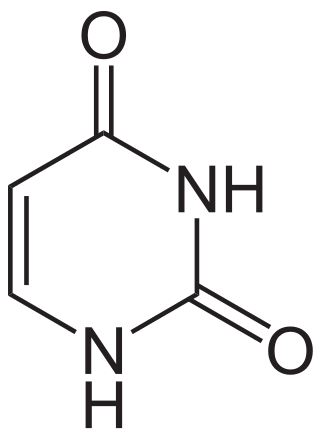
Uracil is one of the four nucleobases in the nucleic acid RNA. The others are adenine (A), cytosine (C), and guanine (G). In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by thymine (T). Uracil is a demethylated form of thymine.
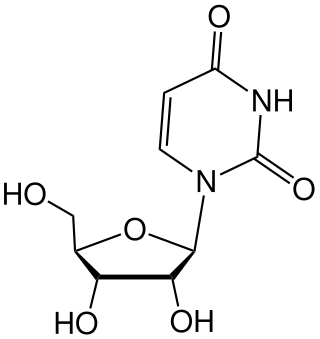
Uridine (symbol U or Urd) is a glycosylated pyrimidine analog containing uracil attached to a ribose ring (or more specifically, a ribofuranose) via a β-N1-glycosidic bond. The analog is one of the five standard nucleosides which make up nucleic acids, the others being adenosine, thymidine, cytidine and guanosine. The five nucleosides are commonly abbreviated to their symbols, U, A, dT, C, and G, respectively. However, thymidine is more commonly written as 'dT' ('d' represents 'deoxy') as it contains a 2'-deoxyribofuranose moiety rather than the ribofuranose ring found in uridine. This is because thymidine is found in deoxyribonucleic acid (DNA) and usually not in ribonucleic acid (RNA). Conversely, uridine is found in RNA and not DNA. The remaining three nucleosides may be found in both RNA and DNA. In RNA, they would be represented as A, C and G whereas in DNA they would be represented as dA, dC and dG.
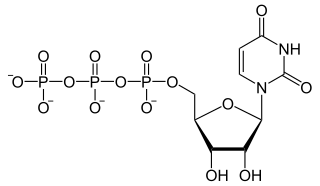
Uridine-5′-triphosphate (UTP) is a pyrimidine nucleoside triphosphate, consisting of the organic base uracil linked to the 1′ carbon of the ribose sugar, and esterified with tri-phosphoric acid at the 5′ position. Its main role is as substrate for the synthesis of RNA during transcription. UTP is the precursor for the production of CTP via CTP synthetase. UTP can be biosynthesized from UDP by Nucleoside Diphosphate Kinase after using the phosphate group from ATP. UDP + ATP ⇌ UTP + ADP; both UTP and ATP are energetically equal.

Uridine diphosphate, abbreviated UDP, is a nucleotide diphosphate. It is an ester of pyrophosphoric acid with the nucleoside uridine. UDP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase uracil.
Glucuronidation is often involved in drug metabolism of substances such as drugs, pollutants, bilirubin, androgens, estrogens, mineralocorticoids, glucocorticoids, fatty acid derivatives, retinoids, and bile acids. These linkages involve glycosidic bonds.

Glucuronic acid is a uronic acid that was first isolated from urine. It is found in many gums such as gum arabic, xanthan, and kombucha tea and is important for the metabolism of microorganisms, plants and animals.

UTP—glucose-1-phosphate uridylyltransferase also known as glucose-1-phosphate uridylyltransferase is an enzyme involved in carbohydrate metabolism. It synthesizes UDP-glucose from glucose-1-phosphate and UTP; i.e.,
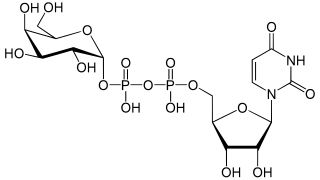
Uridine diphosphate galactose (UDP-galactose) is an intermediate in the production of polysaccharides. It is important in nucleotide sugars metabolism, and is the substrate for the transferase B4GALT5.

Galactose-1-phosphate uridylyltransferase deficiency(classic galactosemia) is the most common type of galactosemia, an inborn error of galactose metabolism, caused by a deficiency of the enzyme galactose-1-phosphate uridylyltransferase. It is an autosomal recessive metabolic disorder that can cause liver disease and death if untreated. Treatment of galactosemia is most successful if initiated early and includes dietary restriction of lactose intake. Because early intervention is key, galactosemia is included in newborn screening programs in many areas. On initial screening, which often involves measuring the concentration of galactose in blood, classic galactosemia may be indistinguishable from other inborn errors of galactose metabolism, including galactokinase deficiency and galactose epimerase deficiency. Further analysis of metabolites and enzyme activities are needed to identify the specific metabolic error.

Uridine diphosphate N-acetylglucosamine or UDP-GlcNAc is a nucleotide sugar and a coenzyme in metabolism. It is used by glycosyltransferases to transfer N-acetylglucosamine residues to substrates. D-Glucosamine is made naturally in the form of glucosamine-6-phosphate, and is the biochemical precursor of all nitrogen-containing sugars. To be specific, glucosamine-6-phosphate is synthesized from fructose 6-phosphate and glutamine as the first step of the hexosamine biosynthesis pathway. The end-product of this pathway is UDP-GlcNAc, which is then used for making glycosaminoglycans, proteoglycans, and glycolipids.
In enzymology, an UDP-arabinose 4-epimerase is an enzyme that catalyzes the chemical reaction

The enzyme UDP-glucose 4-epimerase, also known as UDP-galactose 4-epimerase or GALE, is a homodimeric epimerase found in bacterial, fungal, plant, and mammalian cells. This enzyme performs the final step in the Leloir pathway of galactose metabolism, catalyzing the reversible conversion of UDP-galactose to UDP-glucose. GALE tightly binds nicotinamide adenine dinucleotide (NAD+), a co-factor required for catalytic activity.
In enzymology, an UDP-glucuronate 4-epimerase is an enzyme that catalyzes the chemical reaction
In enzymology, an UDP-glucuronate 5'-epimerase is an enzyme that catalyzes the chemical reaction
In enzymology, a lipopolysaccharide 3-alpha-galactosyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a glucuronate-1-phosphate uridylyltransferase is an enzyme that catalyzes the chemical reaction
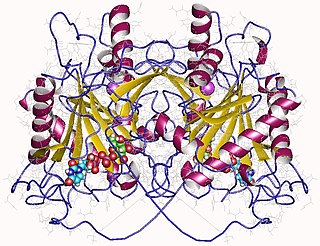
In enzymology, an UDP-glucose—hexose-1-phosphate uridylyltransferase is an enzyme that catalyzes the chemical reaction
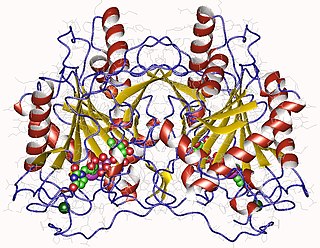
In enzymology, an UTP—hexose-1-phosphate uridylyltransferase is an enzyme that catalyzes the chemical reaction
David Sidney Feingold was an American biochemist.
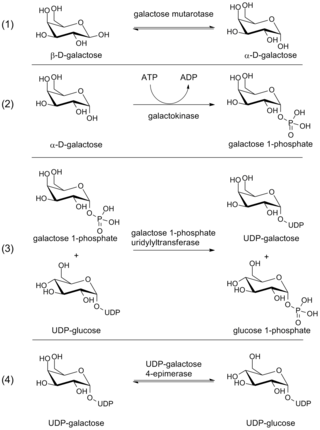
The Leloir pathway is a metabolic pathway for the catabolism of D-galactose. It is named after Luis Federico Leloir, who first described it.













