 | |
| Names | |
|---|---|
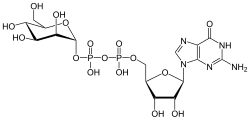
| IUPAC name Guanosine 5′-(α-D-mannopyranosyl dihydrogen diphosphate) | |
| Systematic IUPAC name O1-{[(2R,3S,4R,5R)-5-(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl} O3-[(2R,3S,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] dihydrogen diphosphate | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| MeSH | Guanosine+Diphosphate+Mannose |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C16H25N5O16P2 | |
| Molar mass | 605.341 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Guanosine diphosphate mannose or GDP-mannose is a nucleotide sugar that is a substrate for glycosyltransferase reactions in metabolism. This compound is a substrate for enzymes called mannosyltransferases.
Contents
Known as donor of activated mannose in all glycolytic reactions, GDP-mannose is essential in eukaryotes. [1]