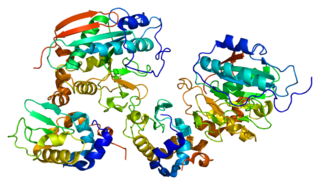Glycoproteins are proteins which contain oligosaccharide chains (glycans) covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated. Carbohydrates are attached to some proteins to form glycoproteins.
A congenital disorder of glycosylation is one of several rare inborn errors of metabolism in which glycosylation of a variety of tissue proteins and/or lipids is deficient or defective. Congenital disorders of glycosylation are sometimes known as CDG syndromes. They often cause serious, sometimes fatal, malfunction of several different organ systems in affected infants. The most common sub-type is PMM2-CDG where the genetic defect leads to the loss of phosphomannomutase 2 (PMM2), the enzyme responsible for the conversion of mannose-6-phosphate into mannose-1-phosphate.

Mannose is a sugar monomer of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation are associated with mutations in enzymes involved in mannose metabolism.

The bacterial outer membrane is found in gram-negative bacteria. Its composition is distinct from that of the inner cytoplasmic cell membrane - among other things, the outer leaflet of the outer membrane of many gram-negative bacteria includes a complex lipopolysaccharide whose lipid portion acts as an endotoxin - and in some bacteria such as E. coli it is linked to the cell's peptidoglycan by Braun's lipoprotein.

Glycosyltransferases are enzymes that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar to a nucleophilic glycosyl acceptor molecule, the nucleophile of which can be oxygen- carbon-, nitrogen-, or sulfur-based.
Transporter associated with antigen processing (TAP) protein complex belongs to the ATP-binding-cassette transporter family. It delivers cytosolic peptides into the endoplasmic reticulum (ER), where they bind to nascent MHC class I molecules.

Colitose is a mannose-derived 3,6-dideoxysugar produced by certain bacteria. It is a constituent of the lipopolysaccharide.

Perosamine is a mannose-derived 4-aminodeoxysugar produced by some bacteria.

The enzyme UDP-glucose 4-epimerase, also known as UDP-galactose 4-epimerase or GALE, is a homodimeric epimerase found in bacterial, fungal, plant, and mammalian cells. This enzyme performs the final step in the Leloir pathway of galactose metabolism, catalyzing the reversible conversion of UDP-galactose to UDP-glucose. GALE tightly binds nicotinamide adenine dinucleotide (NAD+), a co-factor required for catalytic activity.
Nucleotide sugars are the activated forms of monosaccharides. Nucleotide sugars act as glycosyl donors in glycosylation reactions. Those reactions are catalyzed by a group of enzymes called glycosyltransferases.

Guanosine diphosphate mannose or GDP-mannose is a nucleotide sugar that is a substrate for glycosyltransferase reactions in metabolism. This compound is a substrate for enzymes called mannosyltransferases.
In enzymology, a dolichyl-phosphate beta-D-mannosyltransferase is an enzyme that catalyzes the chemical reaction
Beta-1,4-galactosyltransferase 1 is an enzyme that in humans is encoded by the B4GALT1 gene.

CMP-sialic acid transporter is a protein that in humans is encoded by the SLC35A1 gene.

GDP-L-fucose synthetase is an enzyme that in humans is encoded by the TSTA3 gene.
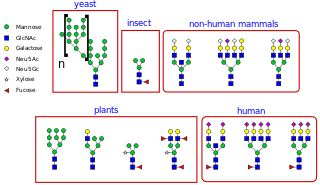
N-linked glycosylation, is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom, in a process called N-glycosylation, studied in biochemistry. This type of linkage is important for both the structure and function of some eukaryotic proteins. The N-linked glycosylation process occurs in eukaryotes and widely in archaea, but very rarely in bacteria. The nature of N-linked glycans attached to a glycoprotein is determined by the protein and the cell in which it is expressed. It also varies across species. Different species synthesize different types of N-linked glycan.
O-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein. O-glycosylation is a post-translational modification that occurs after the protein has been synthesised. In eukaryotes, it occurs in the endoplasmic reticulum, Golgi apparatus and occasionally in the cytoplasm; in prokaryotes, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell metabolism and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including cancer, diabetes and Alzheimer's. O-glycosylation occurs in all domains of life, including eukaryotes, archaea and a number of pathogenic bacteria including Burkholderia cenocepacia, Neisseria gonorrhoeae and Acinetobacter baumannii.
GDP-mannose:cellobiosyl-diphosphopolyprenol alpha-mannosyltransferase is an enzyme with systematic name GDP-mannose:D-Glc-beta-(1->4)-Glc-alpha-1-diphospho-ditrans,octacis-undecaprenol 3-alpha-mannosyltransferase . This enzyme catalyses the following chemical reaction
Transmembrane protein 241 is a ubiquitous sugar transporter protein which in humans is encoded by the TMEM241 gene.
The lysosomal cystine transporter (LCT) family is part of the TOG Superfamily and includes secondary transport proteins that are derived from animals, plants, fungi and other eukaryotes. They exhibit 7 putative transmembrane α-helical spanners (TMSs) and vary in size between about 200 and 500 amino acyl residues, although most have between 300 and 400 residues.