
Cellular differentiation is the process in which a stem cell changes from one type to a differentiated one. Usually, the cell changes to a more specialized type. Differentiation happens multiple times during the development of a multicellular organism as it changes from a simple zygote to a complex system of tissues and cell types. Differentiation continues in adulthood as adult stem cells divide and create fully differentiated daughter cells during tissue repair and during normal cell turnover. Some differentiation occurs in response to antigen exposure. Differentiation dramatically changes a cell's size, shape, membrane potential, metabolic activity, and responsiveness to signals. These changes are largely due to highly controlled modifications in gene expression and are the study of epigenetics. With a few exceptions, cellular differentiation almost never involves a change in the DNA sequence itself. However, metabolic composition does get altered quite dramatically where stem cells are characterized by abundant metabolites with highly unsaturated structures whose levels decrease upon differentiation. Thus, different cells can have very different physical characteristics despite having the same genome.

Bcl-2, encoded in humans by the BCL2 gene, is the founding member of the Bcl-2 family of regulator proteins that regulate cell death (apoptosis), by either inhibiting (anti-apoptotic) or inducing (pro-apoptotic) apoptosis. It was the first apoptosis regulator identified in any organism.

The spindle checkpoint, also known as the metaphase-to-anaphase transition, the spindle assembly checkpoint (SAC), the metaphase checkpoint, or the mitotic checkpoint, is a cell cycle checkpoint during metaphase of mitosis or meiosis that prevents the separation of the duplicated chromosomes (anaphase) until each chromosome is properly attached to the spindle. To achieve proper segregation, the two kinetochores on the sister chromatids must be attached to opposite spindle poles. Only this pattern of attachment will ensure that each daughter cell receives one copy of the chromosome. The defining biochemical feature of this checkpoint is the stimulation of the anaphase-promoting complex by M-phase cyclin-CDK complexes, which in turn causes the proteolytic destruction of cyclins and proteins that hold the sister chromatids together.
Peter G. Schultz is an American chemist. He is the CEO and Professor of Chemistry at The Scripps Research Institute, the founder and former director of GNF, and the founding director of the California Institute for Biomedical Research (Calibr), established in 2012. In August 2014, Nature Biotechnology ranked Schultz the #1 top translational researcher in 2013.
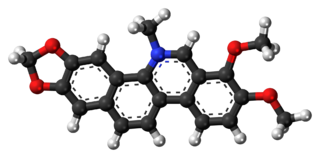
Chelerythrine is a benzophenanthridine alkaloid present in the plant Chelidonium majus. It is a potent, selective, and cell-permeable protein kinase C inhibitor in vitro. And an efficacious antagonist of G-protein-coupled CB1 receptors. This molecule also exhibits anticancer qualities and it has served as a base for many potential novel drugs against cancer. Structurally, this molecule has two distinct conformations, one being a positively charged iminium form, and the other being an uncharged form, a pseudo-base.
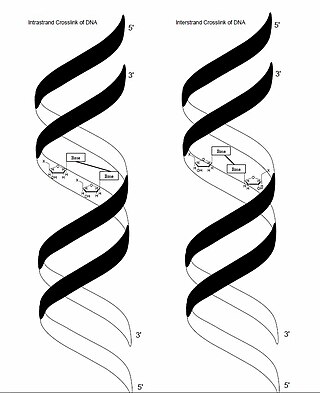
In genetics, crosslinking of DNA occurs when various exogenous or endogenous agents react with two nucleotides of DNA, forming a covalent linkage between them. This crosslink can occur within the same strand (intrastrand) or between opposite strands of double-stranded DNA (interstrand). These adducts interfere with cellular metabolism, such as DNA replication and transcription, triggering cell death. These crosslinks can, however, be repaired through excision or recombination pathways.

U0126 is the 'code' name for a compound associated with cancer treatment and also in preventing ischemia and cellular oxidative stress. It also has likely utility in strokes and heart attacks. This compound is available for research purposes from a number of companies.

Aurora kinase inhibitors are a putative drug class for treating cancer. The Aurora kinase enzymes could be potential targets for novel small-molecule enzyme inhibitors.

Checkpoint kinase 1, commonly referred to as Chk1, is a serine/threonine-specific protein kinase that, in humans, is encoded by the CHEK1 gene. Chk1 coordinates the DNA damage response (DDR) and cell cycle checkpoint response. Activation of Chk1 results in the initiation of cell cycle checkpoints, cell cycle arrest, DNA repair and cell death to prevent damaged cells from progressing through the cell cycle.

Heat shock protein HSP 90-alpha is a protein that in humans is encoded by the HSP90AA1 gene.

Enhancer of zeste homolog 2 (EZH2) is a histone-lysine N-methyltransferase enzyme encoded by EZH2 gene, that participates in histone methylation and, ultimately, transcriptional repression. EZH2 catalyzes the addition of methyl groups to histone H3 at lysine 27, by using the cofactor S-adenosyl-L-methionine. Methylation activity of EZH2 facilitates heterochromatin formation thereby silences gene function. Remodeling of chromosomal heterochromatin by EZH2 is also required during cell mitosis.

Rho-associated protein kinase (ROCK) is a kinase belonging to the AGC family of serine-threonine specific protein kinases. It is involved mainly in regulating the shape and movement of cells by acting on the cytoskeleton.

Ming-Ming Zhou is an American scientist who focuses on structural and chemical biology, NMR spectroscopy, and drug design. He is the Dr. Harold and Golden Lamport Professor and Chairman of the Department of Pharmacological Sciences. He is also the co-director of the Drug Discovery Institute at the Icahn School of Medicine at Mount Sinai and Mount Sinai Health System in New York City, as well as Professor of Sciences. Zhou is an elected fellow of the American Association for the Advancement of Science.

Kinesin-like protein KIF11 is a molecular motor protein that is essential in mitosis. In humans it is coded for by the gene KIF11. Kinesin-like protein KIF11 is a member of the kinesin superfamily, which are nanomotors that move along microtubule tracks in the cell. Named from studies in the early days of discovery, it is also known as Kinesin-5, or as BimC, Eg5 or N-2, based on the founding members of this kinesin family.

Chrysophanol, also known as chrysophanic acid, is a fungal isolate and a natural anthraquinone. It is a C-3 methyl substituted chrysazin of the trihydroxyanthraquinone family.

Rho-kinase inhibitors are a series of compounds that target rho kinase (ROCK) and inhibit the ROCK pathway. Clinical trials have found that inhibition of the ROCK pathway contributes to the cardiovascular benefits of statin therapy. Furthermore, ROCK inhibitors may have clinical applications for anti-erectile dysfunction, antihypertension, and tumor metastasis inhibition. More recently they have been studied for the treatment of glaucoma and as a therapeutic target for the treatment of cardiovascular diseases, including ischemic stroke. While statin therapy has been demonstrated to reduce the risk of major cardiovascular events, including ischemic stroke, the interplay between the ROCK pathway and statin therapy to treat and prevent strokes in older adults has not yet been proven.
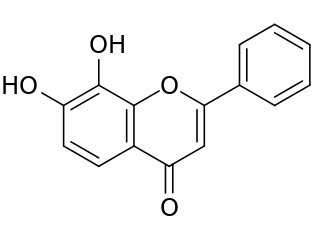
Tropoflavin, also known as 7,8-dihydroxyflavone, is a naturally occurring flavone found in Godmania aesculifolia, Tridax procumbens, and primula tree leaves. It has been found to act as a potent and selective small-molecule agonist of the tropomyosin receptor kinase B (TrkB), the main signaling receptor of the neurotrophin brain-derived neurotrophic factor (BDNF). Tropoflavin is both orally bioavailable and able to penetrate the blood–brain barrier. A prodrug of tropoflavin with greatly improved potency and pharmacokinetics, R13, is under development for the treatment of Alzheimer's disease.
BET inhibitors are a class of drugs that reversibly bind the bromodomains of Bromodomain and Extra-Terminal motif (BET) proteins BRD2, BRD3, BRD4, and BRDT, and prevent protein-protein interaction between BET proteins and acetylated histones and transcription factors.
A proteolysis targeting chimera (PROTAC) is a heterobifunctional molecule composed of two active domains and a linker, capable of removing specific unwanted proteins. Rather than acting as a conventional enzyme inhibitor, a PROTAC works by inducing selective intracellular proteolysis. PROTACs consist of two covalently linked protein-binding molecules: one capable of engaging an E3 ubiquitin ligase, and another that binds to a target protein meant for degradation. Recruitment of the E3 ligase to the target protein results in ubiquitination and subsequent degradation of the target protein via the proteasome. Because PROTACs need only to bind their targets with high selectivity, there are currently many efforts to retool previously ineffective inhibitor molecules as PROTACs for next-generation drugs.
RNA-targeting small molecules represent a class of small molecules, organic compounds with traditional drug properties that can bind to RNA secondary or tertiary structures and alter translation patterns, localization, and degradation.
















