Caffeine is a central nervous system (CNS) stimulant of the methylxanthine class. There are several known mechanisms of action to explain the effects of caffeine. The most prominent is that it reversibly blocks the action of adenosine on its receptors and consequently prevents the onset of drowsiness induced by adenosine. Caffeine also stimulates certain portions of the autonomic nervous system. It is also used as a cognitive enhancer which increases alertness and attentional performance.

A phosphodiesterase inhibitor is a drug that blocks one or more of the five subtypes of the enzyme phosphodiesterase (PDE), thereby preventing the inactivation of the intracellular second messengers, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) by the respective PDE subtype(s). The ubiquitous presence of this enzyme means that non-specific inhibitors have a wide range of actions, the actions in the heart, and lungs being some of the first to find a therapeutic use.

Xanthine is a purine base found in most human body tissues and fluids, as well as in other organisms. Several stimulants are derived from xanthine, including caffeine, theophylline, and theobromine.

Adenosine (symbol A or Ado) is an organic compound that occurs widely in nature in the form of diverse derivatives. The molecule consists of an adenine attached to a ribose via a β-N9-glycosidic bond. Adenosine is one of four nucleoside building blocks to DNA and RNA, which are essential for all life. Its derivatives include the energy carriers adenosine mono-, di-, and triphosphate, also known as AMP/ADP/ATP.

The adenosine receptors (or P1 receptors) are a class of purinergic G protein-coupled receptors with adenosine as the endogenous ligand. There are four known types of adenosine receptors in humans: A1, A2A, A2B and A3; each is encoded by a different gene.

Caffeine dependence is the condition of having a substance dependence on caffeine, a commonplace central nervous system stimulant drug which occurs in nature in coffee, tea, yerba mate, cocoa, and other plants. Caffeine is also an additive in many consumer products, including beverages such as caffeinated alcoholic beverages, energy drinks, colas.

Paraxanthine, or 1,7-dimethylxanthine, is a dimethyl derivative of xanthine, structurally related to caffeine.

An analeptic, in medicine, is a central nervous system stimulant. The term "analeptic" typically refers to respiratory analeptics. Analeptics are central nervous system (CNS) stimulants that include a wide variety of medications used to treat depression, attention deficit hyperactivity disorder (ADHD), and respiratory depression. Analeptics can also be used as convulsants, with low doses causing patients to experience heightened awareness, restlessness, and rapid breathing. The primary medical use of these drugs is as an anesthetic recovery tool or to treat emergency respiratory depression. Other drugs of this category are prethcamide, pentylenetetrazole, and nikethamide. Nikethamide is now withdrawn due to risk of convulsions. Analeptics have recently been used to better understand the treatment of a barbiturate overdose. Through the use of agents, researchers were able to treat obtundation and respiratory depression.

The adenosine A1 receptor is one member of the adenosine receptor group of G protein-coupled receptors with adenosine as endogenous ligand.

The adenosine A2A receptor, also known as ADORA2A, is an adenosine receptor, and also denotes the human gene encoding it.

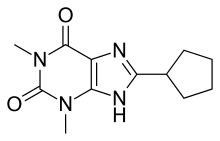
8-Cyclopentyl-1,3-dipropylxanthine (DPCPX, PD-116,948) is a drug which acts as a potent and selective antagonist for the adenosine A1 receptor. It has high selectivity for A1 over other adenosine receptor subtypes, but as with other xanthine derivatives DPCPX also acts as a phosphodiesterase inhibitor, and is almost as potent as rolipram at inhibiting PDE4. It has been used to study the function of the adenosine A1 receptor in animals, which has been found to be involved in several important functions such as regulation of breathing and activity in various regions of the brain, and DPCPX has also been shown to produce behavioural effects such as increasing the hallucinogen-appropriate responding produced by the 5-HT2A agonist DOI, and the dopamine release induced by MDMA, as well as having interactions with a range of anticonvulsant drugs.

SCH-58261 is a drug which acts as a potent and selective antagonist for the adenosine receptor A2A, with more than 50x selectivity for A2A over other adenosine receptors. It has been used to investigate the mechanism of action of caffeine, which is a mixed A1 / A2A antagonist, and has shown that the A2A receptor is primarily responsible for the stimulant and ergogenic effects of caffeine, but blockade of both A1 and A2A receptors is required to accurately replicate caffeine's effects in animals. SCH-58261 has also shown antidepressant, nootropic and neuroprotective effects in a variety of animal models, and has been investigated as a possible treatment for Parkinson's disease.

Istradefylline, sold under the brand name Nourianz, is a medication used as an add-on treatment to levodopa/carbidopa in adults with Parkinson's disease (PD) experiencing "off" episodes. Istradefylline reduces "off" periods resulting from long-term treatment with the antiparkinson drug levodopa. An "off" episode is a time when a patient's medications are not working well, causing an increase in PD symptoms, such as tremor and difficulty walking.

KF-26777 is a drug which acts as a potent and selective antagonist for the adenosine A3 receptor, with sub-nanomolar affinity (A3 Ki=0.2nM) and high selectivity over the other three adenosine receptor subtypes. Simple xanthine derivatives such as caffeine and DPCPX have generally low affinity for the A3 subtype and must be extended by expanding the ring system and adding an aromatic group to give high A3 affinity and selectivity.

PSB-10 is a drug which acts as a selective antagonist for the adenosine A3 receptor (ki value at human A3 receptor is 0.44 nM), with high selectivity over the other three adenosine receptor subtypes (ki values at human A1, A2A and A2B receptors are 4.1, 3.3 and 30 μM). Further pharmacological experiments in a [35S]GTPγS binding assay using hA3-CHO-cells indicated that PSB-10 acts as an inverse agonist (IC50 = 4 nM). It has been shown to produce antiinflammatory effects in animal studies. Simple xanthine derivatives such as caffeine and DPCPX have generally low affinity for the A3 subtype and must be extended by expanding the ring system and adding an aromatic group to give high A3 affinity and selectivity. The affinity towards adenosine A3 subtype was measured against the radioligand PSB-11.

Bamifylline is a drug of the xanthine chemical class which acts as a selective adenosine A1 receptor antagonist.

CGS-15943 is a drug which acts as a potent and reasonably selective antagonist for the adenosine receptors A1 and A2A, having a Ki of 3.3nM at A2A and 21nM at A1. It was one of the first adenosine receptor antagonists discovered that is not a xanthine derivative, instead being a triazoloquinazoline. Consequently, CGS-15943 has the advantage over most xanthine derivatives that it is not a phosphodiesterase inhibitor, and so has more a specific pharmacological effects profile. It produces similar effects to caffeine in animal studies, though with higher potency.
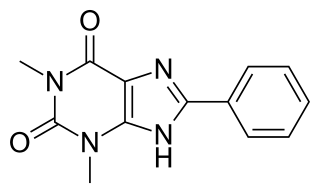
8-Phenyltheophylline (8-phenyl-1,3-dimethylxanthine, 8-PT) is a drug derived from the xanthine family which acts as a potent and selective antagonist for the adenosine receptors A1 and A2A, but unlike other xanthine derivatives has virtually no activity as a phosphodiesterase inhibitor. It has stimulant effects in animals with similar potency to caffeine. Coincidentally 8-phenyltheophylline has also been found to be a potent and selective inhibitor of the liver enzyme CYP1A2 which makes it likely to cause interactions with other drugs which are normally metabolised by CYP1A2.
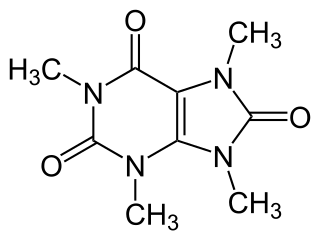
Theacrine, also known as 1,3,7,9-tetramethyluric acid, is a purine alkaloid found in Cupuaçu and in a Chinese tea known as kucha. It shows anti-inflammatory and analgesic effects and appears to affect adenosine signalling in a manner similar to caffeine. In kucha leaves, theacrine is synthesized from caffeine in what is thought to be a three-step pathway.
Caffeine-induced anxiety disorder is a subclass of the DSM-5 diagnosis of substance/medication-induced anxiety disorder.