An anorectic is a drug which reduces appetite, resulting in lower food consumption, leading to weight loss. These substances work by affecting the central nervous system or certain neurotransmitters to create a feeling of fullness or reduce the desire to eat. The understanding of anorexiant effects is crucial in the development of interventions for weight management, eating disorders, and related health concerns. The anorexiant effect can be induced through diverse mechanisms, ranging from hormonal regulation to neural signaling. Ghrelin, leptin, and peptide YY are among the hormones involved in appetite control. Additionally, neurotransmitters such as serotonin and dopamine in the central nervous system contribute significantly to the regulation of food intake.

Fenfluramine, sold under the brand name Fintepla, is a serotonergic medication used for the treatment of seizures associated with Dravet syndrome and Lennox–Gastaut syndrome. It was formerly used as an appetite suppressant in the treatment of obesity, but was discontinued for this use due to cardiovascular toxicity before being repurposed for new indications. Fenfluramine was used for weight loss both alone under the brand name Pondimin and in combination with phentermine commonly known as fen-phen.
Phentermine, sold under the brand name Adipex-P among others, is a medication used together with diet and exercise to treat obesity. It is available by itself or as the combination phentermine/topiramate. Phentermine is taken by mouth.
Thailand's Psychotropic Substances Act is a law designed to regulate certain mind-altering drugs. According to the Office of the Narcotics Control Board, "The Act directly resulted from the Convention on Psychotropic Substances 1971 of which Thailand is a party." The Act divides psychotropic drugs into four Schedules. Offenses involving Schedule I and II drugs carry heavier penalties than those involving Schedule III and IV drugs. Note that this statute does not regulate most opioids, cocaine, or some amphetamines. The vast majority of narcotic painkillers, along with cocaine and most amphetamines are regulated under the Narcotics Act.

Phenmetrazine, sold under the brand name Preludin among others, is a stimulant drug first synthesized in 1952 and originally used as an appetite suppressant, but withdrawn from the market in the 1980s due to widespread misuse. It was initially replaced by its analogue phendimetrazine which functions as a prodrug to phenmetrazine, but now it is rarely prescribed, due to concerns of misuse and addiction. Chemically, phenmetrazine is a substituted amphetamine containing a morpholine ring or a substituted phenylmorpholine.

Aminorex, sold under the brand names Menocil and Apiquel among others, is a weight loss (anorectic) stimulant drug. It was withdrawn from the market after it was found to cause pulmonary hypertension (PPH). In the United States, aminorex is a Schedule I controlled substance.

Chlorphentermine, sold under the brand names Apsedon, Desopimon, and Lucofen, is a serotonergic appetite suppressant of the amphetamine family. Developed in 1962, it is the para-chloro derivative of the better-known appetite suppressant phentermine, which is still in current use.

3,4-Methylenedioxyphentermine (MDPH) is a lesser-known drug of the amphetamine family. MDPH was first synthesized by Alexander Shulgin. Very little data exists about the pharmacological properties, metabolism, and toxicity of MDPH.

Xylopropamine, also known as 3,4-dimethylamphetamine, is a stimulant drug of the phenethylamine and amphetamine classes which was developed and marketed as an appetite suppressant in the 1950s.

Norfenfluramine, or 3-trifluoromethylamphetamine, is a never-marketed drug of the amphetamine family and a major active metabolite of the appetite suppressants fenfluramine and benfluorex. The compound is a racemic mixture of two enantiomers with differing activities, dexnorfenfluramine and levonorfenfluramine.

Mephentermine, sold under the brand name Wyamine among others, is a sympathomimetic medication which was previously used in the treatment of low blood pressure but is mostly no longer marketed. It is used by injection into a vein or muscle, by inhalation, and by mouth.

para-Chloroamphetamine (PCA), also known as 4-chloroamphetamine (4-CA), is a serotonin–norepinephrine–dopamine releasing agent (SNDRA) and serotonergic neurotoxin of the amphetamine family. It is used in scientific research in the study of the serotonin system, as a serotonin releasing agent (SRA) at lower doses to produce serotonergic effects, and as a serotonergic neurotoxin at higher doses to produce long-lasting depletions of serotonin.

A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of one or more monoamine neurotransmitters from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitters and hence enhanced signaling by those neurotransmitters. The monoamine neurotransmitters include serotonin, norepinephrine, and dopamine; MRAs can induce the release of one or more of these neurotransmitters.

A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons, including dopamine and norepinephrine neurons.
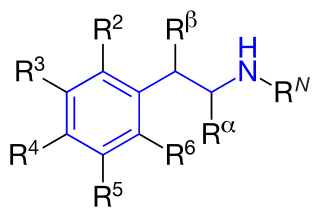
Substituted phenethylamines are a chemical class of organic compounds that are based upon the phenethylamine structure; the class is composed of all the derivative compounds of phenethylamine which can be formed by replacing, or substituting, one or more hydrogen atoms in the phenethylamine core structure with substituents. Phenylethylamines are also generally found to be central nervous system stimulants with many also being entactogens/empathogens, and hallucinogens.

4-Methylmethamphetamine (4-MMA), also known as mephedrine, is a putative stimulant and entactogen drug of the amphetamine family. It acts as a serotonin–norepinephrine–dopamine releasing agent (SNDRA). The drug is the β-deketo analogue of mephedrone and the N-methyl analogue of 4-methylamphetamine (4-MA).

Etolorex is an anorectic of the amphetamine class. It was never marketed.

3,4-Dichloroamphetamine (3,4-DCA), is an amphetamine derived drug invented by Eli Lilly in the 1960s, which has a number of pharmacological actions. It acts as a highly potent and selective serotonin releasing agent (SSRA) and binds to the serotonin transporter with high affinity, but also acts as a selective serotonergic neurotoxin in a similar manner to the related para-chloroamphetamine, though with slightly lower potency. It is also a monoamine oxidase inhibitor (MAOI), as well as a very potent inhibitor of the enzyme phenylethanolamine N-methyl transferase which normally functions to transform noradrenaline into adrenaline in the body.

Cericlamine is a potent and moderately selective serotonin reuptake inhibitor (SSRI) of the amphetamine family that was investigated as an antidepressant for the treatment of depression, anxiety disorders, and anorexia nervosa by Jouveinal but did not complete development and was never marketed. It reached phase III clinical trials in 1996 before development was discontinued in 1999.

2-Phenylmorpholine is the parent compound of the substituted phenylmorpholine class of compounds. Examples of 2-phenylmorpholine derivatives include phenmetrazine (3-methyl-2-phenylmorpholine), phendimetrazine ( -3,4-dimethyl-2-phenylmorpholine), and pseudophenmetrazine ( -3-methyl-2-phenylmorpholine), which are monoamine releasing agents (MRAs) and psychostimulants. 2-Phenylmorpholine itself is a potent norepinephrine–dopamine releasing agent (NDRA) and hence may act as a stimulant similarly.


















