Related Research Articles

The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle, or TCA cycle —is a series of biochemical reactions to release the energy stored in nutrients through the oxidation of acetyl-CoA derived from carbohydrates, fats, proteins, and alcohol. The chemical energy released is available in the form of ATP. The Krebs cycle is used by organisms that respire to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism. Even though it is branded as a "cycle", it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized.

A mitochondrion (pl. mitochondria) is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. Meaning a thread-like granule, the term mitochondrion was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase popularized by Philip Siekevitz in a 1957 Scientific American article of the same name.

Oxidative phosphorylation or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation processes such as anaerobic glycolysis.
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. Many of the enzymes in the electron transport chain are embedded within the membrane.
A proton pump is an integral membrane protein pump that builds up a proton gradient across a biological membrane. Proton pumps catalyze the following reaction:

Cellular respiration is the process by which biological fuels are oxidized in the presence of an inorganic electron acceptor, such as oxygen, to drive the bulk production of adenosine triphosphate (ATP), which contains energy. Cellular respiration may be described as a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from nutrients into ATP, and then release waste products.

Chemiosmosis is the movement of ions across a semipermeable membrane bound structure, down their electrochemical gradient. An important example is the formation of adenosine triphosphate (ATP) by the movement of hydrogen ions (H+) across a membrane during cellular respiration or photosynthesis.

In the mitochondrion, the matrix is the space within the inner membrane. The word "matrix" stems from the fact that this space is viscous, compared to the relatively aqueous cytoplasm. The mitochondrial matrix contains the mitochondrial DNA, ribosomes, soluble enzymes, small organic molecules, nucleotide cofactors, and inorganic ions.[1] The enzymes in the matrix facilitate reactions responsible for the production of ATP, such as the citric acid cycle, oxidative phosphorylation, oxidation of pyruvate, and the beta oxidation of fatty acids.
Thermogenic means tending to produce heat, and the term is commonly applied to drugs which increase heat through metabolic stimulation, or to microorganisms which create heat within organic waste. Approximately all enzymatic reaction in the human body is thermogenic, which gives rise to the basal metabolic rate.
Substrate-level phosphorylation is a metabolism reaction that results in the production of ATP or GTP supported by the energy released from another high-energy bond that leads to phosphorylation of ADP or GDP to ATP or GTP (note that the reaction catalyzed by creatine kinase is not considered as "substrate-level phosphorylation"). This process uses some of the released chemical energy, the Gibbs free energy, to transfer a phosphoryl (PO3) group to ADP or GDP. Occurs in glycolysis and in the citric acid cycle.
In biochemistry and metabolism, beta oxidation (also β-oxidation) is the catabolic process by which fatty acid molecules are broken down in the cytosol in prokaryotes and in the mitochondria in eukaryotes to generate acetyl-CoA. Acetyl-CoA enters the citric acid cycle, generating NADH and FADH2, which are electron carriers used in the electron transport chain. It is named as such because the beta carbon of the fatty acid chain undergoes oxidation and is converted to a carbonyl group to start the cycle all over again. Beta-oxidation is primarily facilitated by the mitochondrial trifunctional protein, an enzyme complex associated with the inner mitochondrial membrane, although very long chain fatty acids are oxidized in peroxisomes.

Oligomycins are macrolides created by Streptomyces that are strong antibacterial agents but are often poisonous to other organisms, including humans.

Carbonyl cyanide m-chlorophenyl hydrazone is a chemical inhibitor of oxidative phosphorylation. It is a nitrile, hydrazone and protonophore. In general, CCCP causes the gradual destruction of living cells and death of the organism, although mild doses inducing partial decoupling have been shown to increase median and maximum lifespan in C. elegans models, suggesting a degree of hormesis. CCCP causes an uncoupling of the proton gradient that is established during the normal activity of electron carriers in the electron transport chain. The chemical acts essentially as an ionophore and reduces the ability of ATP synthase to function optimally. It is routinely used as an experimental uncoupling agent in cell and molecular biology, particularly in the study of mitophagy, where it was integral in discovering the role of the Parkinson's disease-associated ubiquitin ligase Parkin. Outside of its effects on mitochondria, CCCP may also disrupt lysosomal degradation during autophagy.

An uncoupling protein (UCP) is a mitochondrial inner membrane protein that is a regulated proton channel or transporter. An uncoupling protein is thus capable of dissipating the proton gradient generated by NADH-powered pumping of protons from the mitochondrial matrix to the mitochondrial intermembrane space. The energy lost in dissipating the proton gradient via UCPs is not used to do biochemical work. Instead, heat is generated. This is what links UCP to thermogenesis. However, not every type of UCPs are related to thermogenesis. Although UCP2 and UCP3 are closely related to UCP1, UCP2 and UCP3 do not affect thermoregulatory abilities of vertebrates. UCPs are positioned in the same membrane as the ATP synthase, which is also a proton channel. The two proteins thus work in parallel with one generating heat and the other generating ATP from ADP and inorganic phosphate, the last step in oxidative phosphorylation. Mitochondria respiration is coupled to ATP synthesis, but is regulated by UCPs. UCPs belong to the mitochondrial carrier (SLC25) family.
A protonophore, also known as a proton translocator, is an ionophore that moves protons across lipid bilayers or other type of membranes. This would otherwise not occur as protons cations (H+) have positive charge and hydrophilic properties, making them unable to cross without a channel or transporter in the form of a protonophore. Protonophores are generally aromatic compounds with a negative charge, that are both hydrophobic and capable of distributing the negative charge over a number of atoms by π-orbitals which delocalize a proton's charge when it attaches to the molecule. Both the neutral and the charged protonophore can diffuse across the lipid bilayer by passive diffusion and simultaneously facilitate proton transport. Protonophores uncouple oxidative phosphorylation via a decrease in the membrane potential of the inner membrane of mitochondria. They stimulate mitochondria respiration and heat production. Protonophores (uncouplers) are often used in biochemistry research to help explore the bioenergetics of chemiosmotic and other membrane transport processes. It has been reported that the protonophore has antibacterial activity by perturbing bacterial proton motive force.

Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) is an ionophore that is a mobile ion carrier. It is referred to as an uncoupling agent because it disrupts ATP synthesis by transporting hydrogen ions through the mitochondrial membrane before they can be used to provide the energy for oxidative phosphorylation. It is a nitrile and hydrazone. FCCP was first described in 1962 by Heytler.

Dinoseb is a common industry name for 6-sec-butyl-2,4-dinitrophenol, a herbicide in the dinitrophenol family. It is a crystalline orange solid which does not readily dissolve in water. Dinoseb is banned as an herbicide in the European Union (EU) and the United States because of its toxicity.

NADH:ubiquinone reductase (non-electrogenic) (EC 1.6.5.9, NDH-2, ubiquinone reductase, coenzyme Q reductase, dihydronicotinamide adenine dinucleotide-coenzyme Q reductase, DPNH-coenzyme Q reductase, DPNH-ubiquinone reductase, NADH-coenzyme Q oxidoreductase, NADH-coenzyme Q reductase, NADH-CoQ oxidoreductase, NADH-CoQ reductase) is an enzyme with systematic name NADH:ubiquinone oxidoreductase. This enzyme catalyses the following chemical reaction:
Fish acute toxicity syndrome (FATS) is a set of common chemical and functional responses in fish resulting from a short-term, acute exposure to a lethal concentration of a toxicant, a chemical or material that can produce an unfavorable effect in a living organism. By definition, modes of action are characterized by FATS because the combination of common responses that represent each fish acute toxicity syndrome characterize an adverse biological effect. Therefore, toxicants that have the same mode of action elicit similar sets of responses in the organism and can be classified by the same fish acute toxicity syndrome.
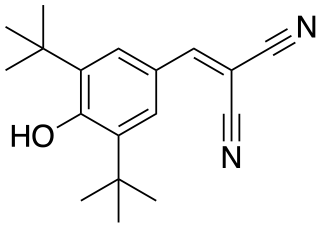
Malonoben is an uncoupling agent/protonophore. As of 1974 when it was discovered, it was considered the most powerful agent of this type, with a potency over 1800 times that of 2,4-dinitrophenol - the prototypical uncoupling agent - and about 3 times the effectiveness of 5-chloro-3-tert-butyl-2'-chloro-4'-nitrosalicylanilide.
References
- 1 2 3 4 Kessler RJ, Tyson CA, Green DE (1976). "Mechanism of uncoupling in mitochondria: Uncouplers as ionophores for cycling cations and protons" (PDF). Proc Natl Acad Sci USA. 73 (9): 3141–3145. Bibcode:1976PNAS...73.3141K. doi: 10.1073/pnas.73.9.3141 . JSTOR 65688. PMC 430958 . PMID 9641.
- ↑ Childress ES, Alexopoulos SJ, Hoehn KS, Santos WL (2018). "Small Molecule Mitochondrial Uncouplers and Their Therapeutic Potential". J Med Chem. 61 (11): 4641–4655. doi:10.1021/acs.jmedchem.7b01182. PMID 29156129.
- ↑ "California Poison Control System: Salicylates". Archived from the original on 2014-08-02.