Alcoholism is the continued drinking of alcohol despite negative results. Problematic use of alcohol has been mentioned in the earliest historical records, the World Health Organization (WHO) estimated there were 283 million people with alcohol use disorders worldwide as of 2016. The term alcoholism was first coined in 1852, but alcoholism and alcoholic are stigmatizing and discourage seeking treatment, so clinical diagnostic terms such as alcohol use disorder or alcohol dependence are used instead.

Methadone, sold under the brand names Dolophine and Methadose among others, is a synthetic opioid agonist used for chronic pain and also for opioid use disorder. It is used to treat chronic pain, and it is also used to treat addiction to heroin or other opioids. Prescribed for daily use, the medicine relieves cravings and removes withdrawal symptoms. Withdrawal management using methadone can be accomplished in less than a month, or it may be done gradually over a longer period of time, or simply maintained for the rest of the patient's life. While a single dose has a rapid effect, maximum effect can take up to five days of use. After long-term use, in people with normal liver function, effects last 8 to 36 hours. Methadone is usually taken by mouth and rarely by injection into a muscle or vein.

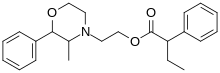
Phendimetrazine is a stimulant drug of the morpholine chemical class used as an appetite suppressant.

Phenmetrazine is a stimulant drug first synthesized in 1952 and originally used as an appetite suppressant, but withdrawn from the market in the 1980s due to widespread abuse. It was initially replaced by its analogue phendimetrazine which functions as a prodrug to phenmetrazine, but now it is rarely prescribed, due to concerns of abuse and addiction. Chemically, phenmetrazine is a substituted amphetamine containing a morpholine ring.

Benzyl benzoate is an organic compound which is used as a medication and insect repellent. As a medication it is used to treat scabies and lice. For scabies either permethrin or malathion is typically preferred. It is applied to the skin as a lotion. Typically two to three applications are needed. It is also present in Balsam of Peru, Tolu balsam, and in a number of flowers.

Etizolam is a thienodiazepine derivative which is a benzodiazepine analog. The etizolam molecule differs from a benzodiazepine in that the benzene ring has been replaced by a thiophene ring and triazole ring has been fused, making the drug a thienotriazolodiazepine.
Most countries have legislation designed to criminalise some drug use. Usually however the legislative process is self-referential, defining abuse in terms of what is made illegal. The legislation concerns lists of drugs specified by the legislation. These drugs are often called illegal drugs but, generally, what is illegal is their unlicensed production, supply and possession. The drugs are also called controlled drugs or controlled substances.

Nicotine dependence is a state of dependence upon nicotine. Nicotine dependence is a chronic, relapsing disease defined as a compulsive craving to use the drug, despite social consequences, loss of control over drug intake, and emergence of withdrawal symptoms. Tolerance is another component of drug dependence. Nicotine dependence develops over time as a person continues to use nicotine. The most commonly used tobacco product is cigarettes, but all forms of tobacco use and e-cigarette use can cause dependence. Nicotine dependence is a serious public health problem because it leads to continued tobacco use, which is one of the leading preventable causes of death worldwide, causing more than 8 million deaths per year.

Oripavine is an opioid and the major metabolite of thebaine. It is the parent compound from which a series of semi-synthetic opioids are derived, which includes the compounds etorphine and buprenorphine. Although its analgesic potency is comparable to morphine, it is not used clinically due to its severe toxicity and low therapeutic index. Due to its use in manufacture of strong opioids, oripavine is a controlled substance in some jurisdictions.
Tapentadol, brand names Nucynta among others, is a centrally acting opioid analgesic of the benzenoid class with a dual mode of action as an agonist of the μ-opioid receptor and as a norepinephrine reuptake inhibitor (NRI). Analgesia occurs within 32 minutes of oral administration, and lasts for 4–6 hours.

The Convention on Psychotropic Substances of 1971 is a United Nations treaty designed to control psychoactive drugs such as amphetamine-type stimulants, barbiturates, benzodiazepines, and psychedelics signed in Vienna, Austria on 21 February 1971. The Single Convention on Narcotic Drugs of 1961 did not ban the many newly discovered psychotropics, since its scope was limited to drugs with cannabis, coca and opium-like effects.

Fenmetramide is a drug which was patented as an antidepressant by McNeil Laboratories in the 1960s. The drug was never marketed. It is the 5-ketone derivative of phenmetrazine and would similarly be expected to produce psychostimulant effects, though pharmacological data is lacking.

G-130 is a drug with stimulant and anorectic effects, related to phenmetrazine.

2-Phenyl-3,6-dimethylmorpholine is a drug with stimulant and anorectic effects, related to phenmetrazine. Based on what is known from other phenylmorpholines with similar structure, it likely acts as a serotonin reuptake inhibitor and may produce antidepressant-like effects. Anecdotal reports suggest, however, that the compound is inactive aside from anorectic effects.

Pseudophenmetrazine is a psychostimulant compound of the morpholine class. It is the N-demethylated and cis-configured analogue of phendimetrazine as well as the cis-configured stereoisomer of phenmetrazine. In addition, along with phenmetrazine, it is believed to be one of the active metabolites of phendimetrazine, which itself is inactive and behaves merely as a prodrug. Relative to phenmetrazine, pseudophenmetrazine is of fairly low potency, acting as a modest releasing agent of norepinephrine (EC50 = 514 nM), while its (+)-enantiomer is a weak releaser of dopamine (EC50 = 1,457 nM) whereas its (−)-enantiomer is a weak reuptake inhibitor of dopamine (Ki = 2,691 nM); together as a racemic mixture with the two enantiomers combined, pseudophenmetrazine behaves overall more as a dopamine reuptake inhibitor (Ki = 2,630 nM), possibly due to the (+)-enantiomer blocking the uptake of the (−)-enantiomer into dopaminergic neurons and thus preventing it from inducing dopamine release. Neither enantiomer has any significant effect on serotonin reuptake or release (both Ki = >10,000 nM and EC50 = >10,000 nM, respectively).

3-Fluorophenmetrazine is a phenylmorpholine-based stimulant and fluorinated analogue of phenmetrazine that has been sold online as a designer drug.
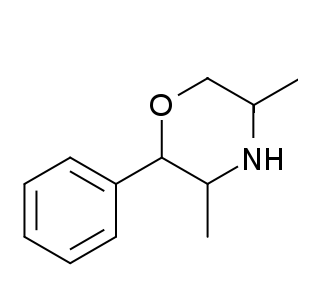
2-Phenyl-3,5-dimethylmorpholine is a drug with stimulant and anorectic effects, related to phenmetrazine. Based on what is known from other phenylmorpholines with similar structure, it likely acts as a norepinephrine-dopamine releasing agent and may produce effects similar or slightly different to phenmetrazine.

4-Methylphenmetrazine is a recreational designer drug with stimulant effects. It is a substituted phenylmorpholine derivative, closely related to better known drugs such as phenmetrazine and 3-fluorophenmetrazine. It was first identified in Slovenia in 2015, and has been shown to act as a monoamine releaser with some preference for serotonin release.
The removal of cannabis and cannabis resin from Schedule IV of the Single Convention on narcotic drugs, 1961 is a change in international law that took place in 2021, on the basis of a scientific assessment by the World Health Organization.