
Phendimetrazine is a stimulant drug of the morpholine chemical class used as an appetite suppressant.

The Controlled Drugs and Substances Act is Canada's federal drug control statute. Passed in 1996 under Prime Minister Jean Chrétien's government, it repeals the Narcotic Control Act and Parts III and IV of the Food and Drugs Act, and establishes eight Schedules of controlled substances and two Classes of precursors. It provides that "The Governor in Council may, by order, amend any of Schedules I to VIII by adding to them or deleting from them any item or portion of an item, where the Governor in Council deems the amendment to be necessary in the public interest."
Ganesha (2,5-dimethoxy-3,4-dimethylamphetamine) is a lesser-known psychedelic drug. It is also a substituted amphetamine. It was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage range is listed as 24–32 mg. The drug is usually taken orally, although other routes such as rectally may also be used. Ganesha is synthesized from 2,5-dimethoxy-3,4-dimethylbenzaldehyde. Ganesha is the amphetamine analog of 2C-G. It is a particularly long lasting drug, with the duration listed in PiHKAL as being 18–24 hours, which might make it undesirable to some users. It is named after the Hindu deity, Ganesha. Very little is known about the dangers or toxicity of ganesha. Effects of ganesha include:
Dimethoxyamphetamine (DMA) is a series of six lesser-known psychedelic drugs similar in structure to the three isomers of methoxyamphetamine and six isomers of trimethoxyamphetamine. The isomers are 2,3-DMA, 2,4-DMA, 2,5-DMA, 2,6-DMA, 3,4-DMA, and 3,5-DMA. Three of the isomers were characterized by Alexander Shulgin in his book PiHKAL. Little is known about their dangers or toxicity.

Xylopropamine, also known as 3,4-dimethylamphetamine, is a stimulant drug of the phenethylamine and amphetamine classes which was developed and marketed as an appetite suppressant in the 1950s.

Dichloropane ((−)-2β-Carbomethoxy-3β-(3,4-dichlorophenyl)tropane, RTI-111, O-401) is a stimulant of the phenyltropane class that acts as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) with IC50 values of 3.13, 18, and 0.79 nM, respectively. In animal studies, dichloropane had a slower onset and longer duration of action compared to cocaine.

Cyclazodone is a centrally acting stimulant drug developed by American Cyanamid Company in the 1960s. The drug is related to other drugs such as pemoline and thozalinone. It displayed a favorable therapeutic index and margin of safety in comparison to Pemoline and other N-lower-alkyl-substituted Pemoline derivatives. The patents concluded that Cyclazodone possessed properties efficacious in reducing fatigue and as a potential anorectic. Structural congeners of Pemoline have been described as "excitants with unique properties distinguishing them from the sympathomimetic amines" whilst displaying less stimulatory activity and toxicity compared to amphetamine.
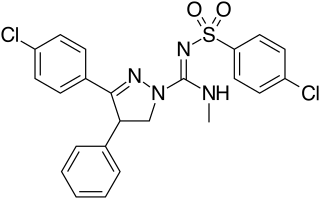
Ibipinabant (SLV319, BMS-646,256) is a drug used in scientific research which acts as a potent and highly selective CB1 antagonist. It has potent anorectic effects in animals, and was researched for the treatment of obesity, although CB1 antagonists as a class have now fallen out of favour as potential anorectics following the problems seen with rimonabant, and so ibipinabant is now only used for laboratory research, especially structure-activity relationship studies into novel CB1 antagonists. SLV330, which is a structural analogue of Ibipinabant, was reported active in animal models related to the regulation of memory, cognition, as well as in addictive behavior. An atom-efficient synthesis of ibipinabant has been reported.

The Decree-Law 15/93 of January 22 is a Portuguese drug control law implementing the 1988 United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances.

SNAP-94847 is a drug used in scientific research, which is a selective, non-peptide antagonist at the melanin concentrating hormone receptor MCH1. In animal studies it has been shown to produce both anxiolytic and antidepressant effects, and also reduces food consumption suggesting a possible anorectic effect.

A-68930 is a synthetic compound that acts as a selective dopamine receptor D1 agonist. It is orally active and has antidepressant and anorectic effects in animals, producing wakefulness and tachycardia, but without stimulant effects, instead producing sedation. The difference in effects between A-68930 and other D1 agonists such as SKF-82958 may be due to their differing effects on the related D5 receptor.
The molecular formula C12H17NO may refer to:

G-130 is a drug with stimulant and anorectic effects, related to phenmetrazine.

Pseudophenmetrazine is a psychostimulant compound of the morpholine class. It is the N-demethylated and cis-configured analogue of phendimetrazine as well as the cis-configured stereoisomer of phenmetrazine. In addition, along with phenmetrazine, it is believed to be one of the active metabolites of phendimetrazine, which itself is inactive and behaves merely as a prodrug. Relative to phenmetrazine, pseudophenmetrazine is of fairly low potency, acting as a modest releasing agent of norepinephrine (EC50 = 514 nM), while its (+)-enantiomer is a weak releaser of dopamine (EC50 = 1,457 nM) whereas its (−)-enantiomer is a weak reuptake inhibitor of dopamine (Ki = 2,691 nM); together as a racemic mixture with the two enantiomers combined, pseudophenmetrazine behaves overall more as a dopamine reuptake inhibitor (Ki = 2,630 nM), possibly due to the (+)-enantiomer blocking the uptake of the (−)-enantiomer into dopaminergic neurons and thus preventing it from inducing dopamine release. Neither enantiomer has any significant effect on serotonin reuptake or release (both Ki = >10,000 nM and EC50 = >10,000 nM, respectively).
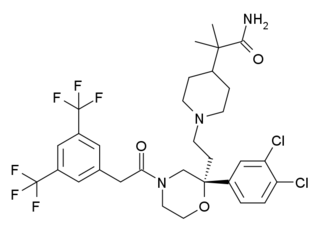
Burapitant (SSR-240,600) is a drug developed by Sanofi-Aventis which was one of the first compounds developed that acts as a potent and selective antagonist for the NK1 receptor. While burapitant itself did not proceed beyond early clinical trials and was never developed for clinical use in humans, promising animal results from this and related compounds have led to a number of novel drugs from this class that have now been introduced into medical use.
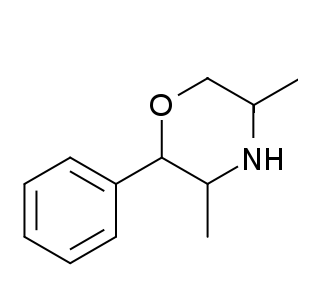
2-Phenyl-3,5-dimethylmorpholine is a drug with stimulant and anorectic effects, related to phenmetrazine. Based on what is known from other phenylmorpholines with similar structure, it likely acts as a norepinephrine-dopamine releasing agent and may produce effects similar or slightly different to phenmetrazine.













