
The N-methyl-D-aspartatereceptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and predominantly Ca2+ ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA and kainate receptors. Depending on its subunit composition, its ligands are glutamate and glycine (or D-serine). However, the binding of the ligands is typically not sufficient to open the channel as it may be blocked by Mg2+ ions which are only removed when the neuron is sufficiently depolarized. Thus, the channel acts as a "coincidence detector" and only once both of these conditions are met, the channel opens and it allows positively charged ions (cations) to flow through the cell membrane. The NMDA receptor is thought to be very important for controlling synaptic plasticity and mediating learning and memory functions.

Zanamivir is a medication used to treat and prevent influenza caused by influenza A and influenza B viruses. It is a neuraminidase inhibitor and was developed by the Australian biotech firm Biota Holdings. It was licensed to Glaxo in 1990 and approved in the US in 1999, only for use as a treatment for influenza. In 2006, it was approved for prevention of influenza A and B. Zanamivir was the first neuraminidase inhibitor commercially developed. It is marketed by GlaxoSmithKline under the trade name Relenza as a powder for oral inhalation.

Rimantadine is an orally administered antiviral drug used to treat, and in rare cases prevent, influenzavirus A infection. When taken within one to two days of developing symptoms, rimantadine can shorten the duration and moderate the severity of influenza. Rimantadine can mitigate symptoms, including fever. Both rimantadine and the similar drug amantadine are derivates of adamantane. Rimantadine is found to be more effective than amantadine because when used the patient displays fewer symptoms. Rimantadine was approved by the Food and Drug Administration (FDA) in 1994.

Amantadine, sold under the brand name Gocovri among others, is a medication used to treat dyskinesia associated with parkinsonism and influenza caused by type A influenzavirus, though its use for the latter is no longer recommended because of widespread drug resistance. It acts as a nicotinic antagonist, dopamine agonist, and noncompetitive NMDA antagonist. The antiviral mechanism of action is antagonism of the influenzavirus A M2 proton channel, which prevents endosomal escape.
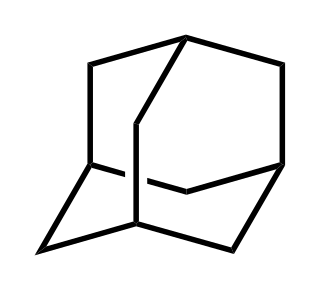
Adamantane is an organic compound with formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond). It is a white solid with a camphor-like odor. It is the simplest diamondoid.

The Matrix-2 (M2) protein is a proton-selective viroporin, integral in the viral envelope of the influenza A virus. The channel itself is a homotetramer, where the units are helices stabilized by two disulfide bonds, and is activated by low pH. The M2 protein is encoded on the seventh RNA segment together with the M1 protein. Proton conductance by the M2 protein in influenza A is essential for viral replication.

Taribavirin is an antiviral drug in Phase III human trials, but not yet approved for pharmaceutical use. It is a prodrug of ribavirin, active against a number of DNA and RNA viruses. Taribavirin has better liver-targeting than ribavirin, and has a shorter life in the body due to less penetration and storage in red blood cells. It is expected eventually to be the drug of choice for viral hepatitis syndromes in which ribavirin is active. These include hepatitis C and perhaps also hepatitis B and yellow fever.

Treatments for influenza include a range of medications and therapies that are used in response to disease influenza. Treatments may either directly target the influenza virus itself; or instead they may just offer relief to symptoms of the disease, while the body's own immune system works to recover from infection.

Umifenovir, sold under the brand name Arbidol, is sold and used as an antiviral medication for influenza in Russia and China. The drug is manufactured by Pharmstandard. It is not approved by the U.S. Food and Drug Administration (FDA) for the treatment or prevention of influenza.

Bifemelane (INN) (Alnert, Celeport), or bifemelane hydrochloride (JAN), also known as 4-(O-benzylphenoxy)-N-methylbutylamine, is an antidepressant and cerebral activator that was widely used in the treatment of cerebral infarction patients with depressive symptoms in Japan, and in the treatment of senile dementia as well. It also appears to be useful in the treatment of glaucoma. It has been discontinued in Japan since 1998, when it was removed from the market reportedly for lack of effectiveness.

Bromantane, sold under the brand name Ladasten, is an atypical psychostimulant and anxiolytic drug of the adamantane family related to amantadine and memantine which is used in Russia in the treatment of neurasthenia. Although the effects of the bromantane have been determined to be dependent on the dopaminergic and possibly serotonergic neurotransmitter systems, its exact mechanism of action is unknown, and it is distinct in its properties relative to typical psychostimulants such as amphetamine. Because of its unique aspects, bromantane has sometimes been described instead as an actoprotector.

Cinazepam is an atypical benzodiazepine derivative. It produces pronounced hypnotic, sedative, and anxiolytic effects with minimal myorelaxant side effects. In addition, unlike many other benzodiazepine and nonbenzodiazepine hypnotics such as diazepam, flunitrazepam, and zopiclone, cinazepam does not violate sleep architecture, and the continuity of slow-wave sleep and REM sleep are proportionally increased. As such, cinazepam produces a sleep state close to physiological, and for that reason, may be advantageous compared to other, related drugs in the treatment of insomnia and other sleep disorders.

Riamilovir is a broad-spectrum antiviral drug developed in Russia through a joint effort of Ural Federal University, Russian Academy of Sciences, Ural Center for Biopharma Technologies and Medsintez Pharmaceutical. It has a novel triazolotriazine core, which represents a new structural class of non-nucleoside antiviral drugs.
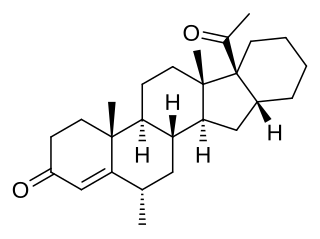
Mecigestone, also known as pentarane B, as well as 6α-methyl-16α,17α-cyclohexanoprogesterone, 6α-methylcyclohexano[1',2';16,17]pregn-4-ene-3,20-dione, or 17α-acetyl-6α-methyl-16β,24-cyclo-21-norchol-4-en-3-one, is a steroidal progestin that was developed by the Zelinskii Institute of Organic Chemistry of the Russian Academy of Sciences and has been proposed for clinical use as a progestogen but has not been marketed. It is the 6α-methylated analogue of pentarane A, which is also known as D'6-pentarane or pregna-D'6-pentarane.
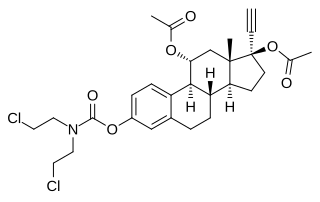
Cytestrol acetate is a steroidal antiestrogen and a cytostatic antineoplastic agent which was developed for the treatment of breast cancer but was never marketed.

Cortifen, also known as cortiphen or kortifen, as well as fencoron, is a synthetic glucocorticoid corticosteroid and cytostatic antineoplastic agent which was developed in Russia for potential treatment of tumors. It is a hydrophobic chlorphenacyl nitrogen mustard ester of 11-deoxycortisol (cortodoxone).
Actoprotectors or synthetic adaptogens are compounds that enhance an organism's resilience to physical stress without increasing heat output. Actoprotectors are distinct from other performance-enhancing substances in that they increase physical and psychological resilience via non-exhaustive action. The term "actoprotector" is used to describe synthetic and isolated compounds possessing adaptogenic properties. By contrast, the term "adaptogen" is most often use to describe a natural herb as a whole, which can contain hundreds if not thousands of biologically active components.

Ethylestradiol, or 17α-ethylestradiol, also known as 17α-ethylestra-1,3,5(10)-triene-3,17β-diol, is a synthetic estrogen which was never marketed. It occurs as an active metabolite of the anabolic steroids norethandrolone and ethylestrenol formed via aromatase and is believed to be responsible for the estrogenic effects of norethandrolone and ethylestrenol. The 3-methyl ether of ethylestradiol has been used as an intermediate in the synthesis of certain 19-nortestosterone anabolic steroids.

Megestrol caproate, abbreviated as MGC, is a progestin medication which was never marketed. It was developed in Russia in 2002. In animals, MGC shows 10-fold higher progestogenic activity compared to progesterone when both are administered via subcutaneous injection. In addition, MGC has no androgenic, anabolic, or estrogenic activity. The medication was suggested as a potential contraceptive and therapeutic agent.

4-Aminoacetanilide or paracetamin is a chemical compound which is a amino derivative of acetanilide and para-isomer of aminoacetanilide. There are two other isomers of aminoacetanilide, 2-aminoacetanilide and 3-aminoacetanilide. Aminoacetanilide derivatives are important synthetic intermediates in heterocyclic and aromatic synthesis. These derivatives have found applications in pharmaceutical industry and dyes and pigment industry.




















