
Ribavirin, also known as tribavirin, is an antiviral medication used to treat RSV infection, hepatitis C and some viral hemorrhagic fevers. For hepatitis C, it is used in combination with other medications such as simeprevir, sofosbuvir, peginterferon alfa-2b or peginterferon alfa-2a. Among the viral hemorrhagic fevers it is sometimes used for Lassa fever, Crimean–Congo hemorrhagic fever, and Hantavirus infection but should not be used for Ebola or Marburg infections. Ribavirin is taken orally or inhaled. Despite widespread usage, since the 2010s it has faced scrutiny for a lack of efficacy in treating viral infections it has historically been prescribed for.
Reverse-transcriptase inhibitors (RTIs) are a class of antiretroviral drugs used to treat HIV infection or AIDS, and in some cases hepatitis B. RTIs inhibit activity of reverse transcriptase, a viral DNA polymerase that is required for replication of HIV and other retroviruses.

Vidarabine or 9-β-D-arabinofuranosyladenine (ara-A) is an antiviral drug which is active against herpes simplex and varicella zoster viruses.

Nucleoside analogues are structural analogues of a nucleoside, which normally contain a nucleobase and a sugar. Nucleotide analogues are analogues of a nucleotide, which normally has one to three phosphates linked to a nucleoside. Both types of compounds can deviate from what they mimick in a number of ways, as changes can be made to any of the constituent parts. They are related to nucleic acid analogues.

Brivudine is an antiviral drug used in the treatment of herpes zoster ("shingles"). Like other antivirals, it acts by inhibiting replication of the target virus.

The Rega Institute for Medical Research is a Belgian scientific establishment that is part of the Catholic University of Leuven (Leuven) in central Belgium. The Rega Institute is an interfacultary biomedical research institute of the Catholic University of Leuven and consists of departments of medicine and pharmacology.

Tiazofurin is a drug which acts as an inhibitor of the enzyme IMP dehydrogenase. Tiazofurin and its analogues were under investigation for potential use in the treatment of cancer, though side effects such as pleuropericarditis and a flu-like syndrome precluded further development. They also show antiviral effects and may be reevaluated as potential options in the treatment of newly emerging viral diseases.
Non-nucleoside reverse-transcriptase inhibitors (NNRTIs) are antiretroviral drugs used in the treatment of human immunodeficiency virus (HIV). NNRTIs inhibit reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of HIV. RT is one of the most popular targets in the field of antiretroviral drug development.
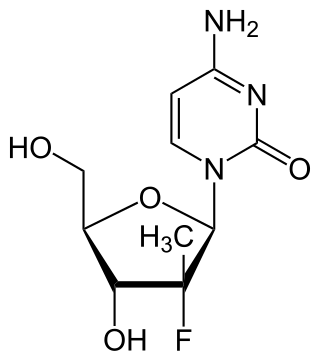
PSI-6130 is an experimental treatment for hepatitis C. PSI-6130 is a member of a class of antiviral drugs known as nucleoside polymerase inhibitors that was created by chemist Jeremy L. Clark. Specifically, PSI-6130 inhibits the hepatitis C virus RNA dependant RNA polymerase called NS5B.
Discovery and development of nucleoside and nucleotide reverse-transcriptase inhibitors began in the 1980s when the AIDS epidemic hit Western societies. NRTIs inhibit the reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of the human immunodeficiency virus (HIV). The first NRTI was zidovudine, approved by the U.S. Food and Drug Administration (FDA) in 1987, which was the first step towards treatment of HIV. Six NRTI agents and one NtRTI have followed. The NRTIs and the NtRTI are analogues of endogenous 2´-deoxy-nucleoside and nucleotide. Drug-resistant viruses are an inevitable consequence of prolonged exposure of HIV-1 to anti-HIV drugs.

PMEG is an acyclic nucleoside phosphonate. Acyclic nucleoside phosphonates can have significant antiviral, cytostatic and antiproliferative activities. PMEG can inhibit cell proliferation and cause genotoxicity. PMEG is active against leukemia and melanoma in animal tumor models, and also has antiviral activities against herpes viruses in murine models.

S2242 is an experimental antiviral agent that is an inhibitor of herpes virus replication.

The ProTide technology is a prodrug approach used in molecular biology and drug design. It is designed to deliver nucleotide analogues into the cell. This technology was invented by Professor Chris McGuigan from the School of Pharmacy and Pharmaceutical Sciences at Cardiff University in the early 1990s. ProTides form a critical part of antiviral drugs such as sofosbuvir, tenofovir alafenamide, and remdesivir.

Carbocyclic nucleosides are nucleoside analogues in which a methylene group has replaced the oxygen atom of the furanose ring. These analogues have the nucleobase attached at a simple alkyl carbon rather than being part of a hemiaminal ether linkage. As a result, they have increased chemical stability. They also have increased metabolic stability because they are unaffected by phosphorylases and hydrolases that cleave the glycosidic bond between the nucleobase and furanose ring of nucleosides. They retain many of the biological properties of the original nucleosides with respect to recognition by various enzymes and receptors.

MK-608 is an antiviral drug, an adenosine analog. It was originally developed by Merck & Co. as a treatment for hepatitis C, but despite promising results in animal studies, it was ultimately unsuccessful in clinical trials. Subsequently it has been widely used in antiviral research and has shown activity against a range of viruses, including Dengue fever, tick-borne encephalitis virus, poliovirus, and most recently Zika virus, in both in vitro and animal models. Since it has already failed in human clinical trials previously, it is unlikely MK-608 itself will be developed as an antiviral medication, but the continuing lack of treatment options for these emerging viral diseases means that much research continues using MK-608 and related antiviral drugs.

Brequinar (DuP-785) is a drug that acts as a potent and selective inhibitor of the enzyme dihydroorotate dehydrogenase. It blocks synthesis of pyrimidine based nucleotides in the body and so inhibits cell growth. Brequinar was invented by DuPont Pharmaceuticals in the 1980s. In 2001, Bristol-Myers Squibb acquired DuPont, and in 2017, Clear Creek Bio acquired the rights to brequinar from BMS.

Pyrazofurin (pyrazomycin) is a natural product found in Streptomyces candidus, which is a nucleoside analogue related to ribavirin. It has antibiotic, antiviral and anti-cancer properties but was not successful in human clinical trials due to severe side effects. Nevertheless, it continues to be the subject of ongoing research as a potential drug of last resort, or a template for improved synthetic derivatives.
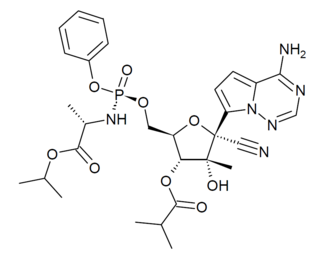
GS-6620 is an antiviral drug which is a nucleotide analogue. It was developed for the treatment of Hepatitis C but while it showed potent antiviral effects in early testing, it could not be successfully formulated into an oral dosage form due to low and variable absorption in the intestines which made blood levels unpredictable. It has however continued to be researched as a potential treatment for other viral diseases such as Ebola virus disease.
Katherine Seley-Radtke is an American medicinal chemist who specializes in the discovery and design of novel nucleoside or nucleotide based enzyme inhibitors that may be used to treat infections or cancer. She has authored over 90 peer-reviewed publications, is an inventor of five issued US patents, and is a professor in the department of chemistry and biochemistry at the University of Maryland, Baltimore County. Her international impact includes scientific collaborations, policy advising and diplomatic appointments in biosecurity efforts.

Sangivamycin is a natural product originally isolated from Streptomyces rimosus, which is a nucleoside analogue. It acts as an inhibitor of protein kinase C. It has antibiotic, antiviral and anti-cancer properties and has been investigated for various medical applications, though never approved for clinical use itself. However, a number of related derivatives continue to be researched.
















