
Hepatitis C is an infectious disease caused by the hepatitis C virus (HCV) that primarily affects the liver; it is a type of viral hepatitis. During the initial infection period, people often have mild or no symptoms. Early symptoms can include fever, dark urine, abdominal pain, and yellow tinged skin. The virus persists in the liver, becoming chronic, in about 70% of those initially infected. Early on, chronic infection typically has no symptoms. Over many years however, it often leads to liver disease and occasionally cirrhosis. In some cases, those with cirrhosis will develop serious complications such as liver failure, liver cancer, or dilated blood vessels in the esophagus and stomach.

Ribavirin, also known as tribavirin, is an antiviral medication used to treat RSV infection, hepatitis C and some viral hemorrhagic fevers. For hepatitis C, it is used in combination with other medications such as simeprevir, sofosbuvir, peginterferon alfa-2b or peginterferon alfa-2a. Among the viral hemorrhagic fevers it is sometimes used for Lassa fever, Crimean–Congo hemorrhagic fever, and Hantavirus infection but should not be used for Ebola or Marburg infections. Ribavirin is taken by mouth or inhaled. Despite widespread usage, since the 2010s it has faced scrutiny for a lack of efficacy in treating viral infections it has historically been prescribed for.

Taribavirin is an antiviral drug in Phase III human trials, but not yet approved for pharmaceutical use. It is a prodrug of ribavirin, active against a number of DNA and RNA viruses. Taribavirin has better liver-targeting than ribavirin, and has a shorter life in the body due to less penetration and storage in red blood cells. It is expected eventually to be the drug of choice for viral hepatitis syndromes in which ribavirin is active. These include hepatitis C and perhaps also hepatitis B and yellow fever.
Pegylated interferon alfa-2b is a drug used to treat melanoma, as an adjuvant therapy to surgery. Also used to treat hepatitis C, it is no longer recommended due to poor efficacy and adverse side-effects. Subcutaneous injection is the preferred delivery method.

Boceprevir is a protease inhibitor used to treat hepatitis caused by hepatitis C virus (HCV) genotype 1. It binds to the HCV nonstructural protein 3 active site.

Sofosbuvir, sold under the brand name Sovaldi among others, is a medication used to treat hepatitis C. It is taken by mouth.
Daclatasvir, sold under the brand name Daklinza, is an antiviral medication used in combination with other medications to treat hepatitis C (HCV). The other medications used in combination include sofosbuvir, ribavirin, and interferon, vary depending on the virus type and whether the person has cirrhosis. It is taken by mouth.

Simeprevir, sold under the brand name Olysio among others, is a medication used in combination with other medications for the treatment of hepatitis C. It is specifically used for hepatitis C genotype 1 and 4. Medications it is used with include sofosbuvir or ribavirin and peginterferon-alfa. Cure rates are in 80s to 90s percent. It may be used in those who also have HIV/AIDS. It is taken by mouth once daily for typically 12 weeks.
Ledipasvir is a drug for the treatment of hepatitis C that was developed by Gilead Sciences. After completing Phase III clinical trials, on February 10, 2014, Gilead filed for U.S. approval of a ledipasvir/sofosbuvir fixed-dose combination tablet for genotype 1 hepatitis C. The ledipasvir/sofosbuvir combination is a direct-acting antiviral agent that interferes with HCV replication and can be used to treat patients with genotypes 1a or 1b without PEG-interferon or ribavirin.
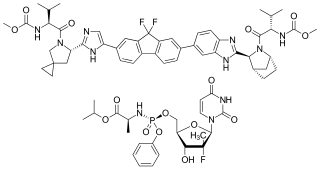
Ledipasvir/sofosbuvir, sold under the trade name Harvoni among others, is a medication used to treat hepatitis C. It is a fixed-dose combination of ledipasvir and sofosbuvir. Cure rates are 94% to 99% in people infected with hepatitis C virus (HCV) genotype 1. Some evidence also supports use in HCV genotype 3 and 4. It is taken daily by mouth for 8–24 weeks.
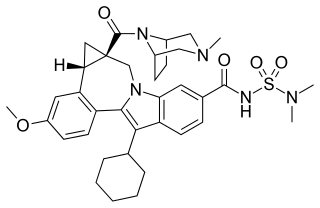
Beclabuvir is an antiviral drug for the treatment of hepatitis C virus (HCV) infection that has been studied in clinical trials. In February 2017, Bristol-Myers Squibb began sponsoring a post-marketing trial of beclabuvir, in combination with asunaprevir and daclatasvir, to study the combination's safety profile with regard to liver function. From February 2014 to November 2016, a phase II clinical trial was conducted on the combination of asunaprevir/daclatasvir/beclabuvir on patients infected with both HIV and HCV. Furthermore, a recent meta-analysis of six published six clinical trials showed high response rates in HCV genotype 1-infected patients treated with daclatasvir, asunaprevir, and beclabuvir irrespective of ribavirin use, prior interferon-based therapy, or restriction on noncirrhotic patients, IL28B genotype, or baseline resistance-associated variants
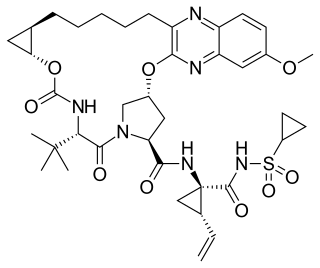
Grazoprevir is a drug approved for the treatment of hepatitis C. It was developed by Merck and completed Phase III trials, used in combination with the NS5A replication complex inhibitor elbasvir under the trade name Zepatier, either with or without ribavirin.
Samatasvir (IDX-719) is an experimental drug for the treatment of hepatitis C. It was originally developed by Idenix, and development has been continued by Merck & Co. following their acquisition of Idenix. Samatasvir has shown good results in Phase II trials.
Elbasvir/grazoprevir, sold under the brand name Zepatier, is a fixed-dose combination for the treatment of hepatitis C, containing elbasvir and grazoprevir. It is used to treat chronic hepatitis C virus (HCV) genotypes 1 or 4 infection in both treatment-naïve and treatment-experienced patients.

MK-608 is an antiviral drug, an adenosine analog. It was originally developed by Merck & Co. as a treatment for hepatitis C, but despite promising results in animal studies, it was ultimately unsuccessful in clinical trials. Subsequently it has been widely used in antiviral research and has shown activity against a range of viruses, including Dengue fever, tick-borne encephalitis virus, poliovirus, and most recently Zika virus, in both in vitro and animal models. Since it has already failed in human clinical trials previously, it is unlikely MK-608 itself will be developed as an antiviral medication, but the continuing lack of treatment options for these emerging viral diseases means that much research continues using MK-608 and related antiviral drugs.

Nonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a culmination of increased understanding of the viral life cycle combined with advances in drug discovery technology. However, their mechanism of action is complex and not fully understood. NS5A inhibitors were the focus of much attention when they emerged as a part of the first curative treatment for hepatitis C virus (HCV) infections in 2014. Favorable characteristics have been introduced through varied structural changes, and structural similarities between NS5A inhibitors that are clinically approved are readily apparent. Despite the recent introduction of numerous new antiviral drugs, resistance is still a concern and these inhibitors are therefore always used in combination with other drugs.
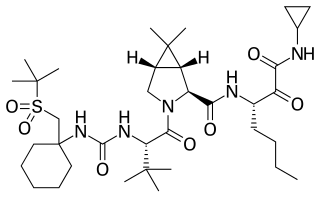
Narlaprevir, is an inhibitor of NS3/4A serine protease, intended for the treatment of chronic hepatitis C caused by genotype 1 virus in combination with other antiviral drugs.

Non-structural protein 5B (NS5B) inhibitors are a class of direct-acting antivirals widely used in the treatment of chronic hepatitis C. Depending on site of action and chemical composition, NS5B inhibitors may be categorized into three classes—nucleoside active site inhibitors (NIs), non-nucleoside allosteric inhibitors, and pyrophosphate analogues. Subsequently, all three classes are then subclassified. All inhibit RNA synthesis by NS5B but at different stages/sites resulting in inability of viral RNA replication. Expression of direct-acting NS5B inhibitors does not take place in cells that are not infected by hepatitis C virus, which seems to be beneficial for this class of drugs.
The term Direct-acting antivirals (DAA) has long been associated with the combination of antiviral drugs used to treat hepatitis C infections. These are the more effective than older treatments such as ribavirin and interferon. The DAA drugs against hepatitis C are taken orally, as tablets, for 8 to 12 weeks. The treatment depends on the type or types (genotypes) of hepatitis C virus that are causing the infection. Both during and at the end of treatment, blood tests are used to monitor the effectiveness of the treatment and subsequent cure.

Bemnifosbuvir is an antiviral drug invented by Atea Pharmaceuticals and licensed to Roche for clinical development, a novel nucleotide analog prodrug originally developed for the treatment of hepatitis C. Bemnifosbuvir is the orally bioavailable hemisulfate salt of AT-511, which is metabolized in several steps to the active nucleotide triphosphate AT-9010, acting as an RNA polymerase inhibitor and thereby interfering with viral replication. Bemnifosbuvir has been researched for the treatment of coronavirus diseases such as that produced by SARS-CoV-2. It showed good results in early clinical trials but had inconsistent results at later stages, so the planned Phase 3 trials are being redesigned and results are not expected until late 2022.














