Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are a class of antimicrobials, a larger group which also includes antibiotic, antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from virucides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural virucides are produced by some plants such as eucalyptus and Australian tea trees.
Protease inhibitors (PIs) are medications that act by interfering with enzymes that cleave proteins. Some of the most well known are antiviral drugs widely used to treat HIV/AIDS, hepatitis C and COVID-19. These protease inhibitors prevent viral replication by selectively binding to viral proteases and blocking proteolytic cleavage of protein precursors that are necessary for the production of infectious viral particles.

Nonstructural protein 5A (NS5A) is a zinc-binding and proline-rich hydrophilic phosphoprotein that plays a key role in Hepatitis C virus RNA replication. It appears to be a dimeric form without trans-membrane helices.

Sofosbuvir, sold under the brand name Sovaldi among others, is a medication used to treat hepatitis C. It is taken by mouth.
Daclatasvir, sold under the brand name Daklinza, is an antiviral medication used in combination with other medications to treat hepatitis C (HCV). The other medications used in combination include sofosbuvir, ribavirin, and interferon, vary depending on the virus type and whether the person has cirrhosis. It is taken by mouth.

Simeprevir, sold under the brand name Olysio among others, is a medication used in combination with other medications for the treatment of hepatitis C. It is specifically used for hepatitis C genotype 1 and 4. Medications it is used with include sofosbuvir or ribavirin and peginterferon-alfa. Cure rates are in 80s to 90s percent. It may be used in those who also have HIV/AIDS. It is taken by mouth once daily for typically 12 weeks.
Ledipasvir is a drug for the treatment of hepatitis C that was developed by Gilead Sciences. After completing Phase III clinical trials, on February 10, 2014, Gilead filed for U.S. approval of a ledipasvir/sofosbuvir fixed-dose combination tablet for genotype 1 hepatitis C. The ledipasvir/sofosbuvir combination is a direct-acting antiviral agent that interferes with HCV replication and can be used to treat patients with genotypes 1a or 1b without PEG-interferon or ribavirin.
Ombitasvir is an antiviral drug for the treatment of hepatitis C virus (HCV) infection by AbbVie. In the United States, it is approved by the Food and Drug Administration for use in combination with paritaprevir, ritonavir and dasabuvir in the product Viekira Pak for the treatment of HCV genotype 1, and with paritaprevir and ritonavir in the product Technivie for the treatment of HCV genotype 4.
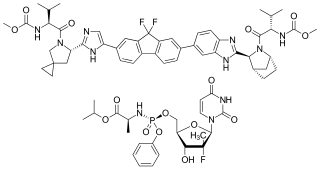
Ledipasvir/sofosbuvir, sold under the trade name Harvoni among others, is a medication used to treat hepatitis C. It is a fixed-dose combination of ledipasvir and sofosbuvir. Cure rates are 94% to 99% in people infected with hepatitis C virus (HCV) genotype 1. Some evidence also supports use in HCV genotype 3 and 4. It is taken daily by mouth for 8–24 weeks.
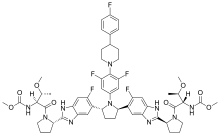
Odalasvir is an investigational new drug in development for the treatment of hepatitis C. It is an NS5A inhibitor. The NS5A protein serves multiple functions at various stages of the viral life cycle, including viral replication. NS5A also plays a role in the development of interferon-resistance, a common cause of treatment failure. It is under development by Achillion Pharmaceuticals.
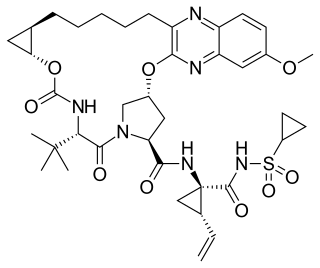
Grazoprevir is a drug approved for the treatment of hepatitis C. It was developed by Merck and completed Phase III trials, used in combination with the NS5A replication complex inhibitor elbasvir under the trade name Zepatier, either with or without ribavirin.

Elbasvir is a drug approved by the FDA in January 2016 for the treatment of hepatitis C. It was developed by Merck and completed Phase III trials, used in combination with the NS3/4A protease inhibitor grazoprevir under the trade name Zepatier, either with or without ribavirin.
Samatasvir (IDX-719) is an experimental drug for the treatment of hepatitis C. It was originally developed by Idenix, and development has been continued by Merck & Co. following their acquisition of Idenix. Samatasvir has shown good results in Phase II trials.
Elbasvir/grazoprevir, sold under the brand name Zepatier, is a fixed-dose combination for the treatment of hepatitis C, containing elbasvir and grazoprevir. It is used to treat chronic hepatitis C virus (HCV) genotypes 1 or 4 infection in both treatment-naïve and treatment-experienced patients.

Nonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a culmination of increased understanding of the viral life cycle combined with advances in drug discovery technology. However, their mechanism of action is complex and not fully understood. NS5A inhibitors were the focus of much attention when they emerged as a part of the first curative treatment for hepatitis C virus (HCV) infections in 2014. Favorable characteristics have been introduced through varied structural changes, and structural similarities between NS5A inhibitors that are clinically approved are readily apparent. Despite the recent introduction of numerous new antiviral drugs, resistance is still a concern and these inhibitors are therefore always used in combination with other drugs.
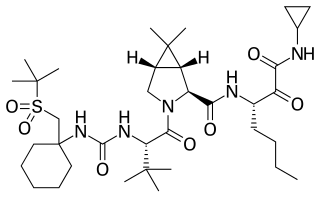
Narlaprevir, is an inhibitor of NS3/4A serine protease, intended for the treatment of chronic hepatitis C caused by genotype 1 virus in combination with other antiviral drugs.

Glecaprevir (INN,) is a hepatitis C virus (HCV) nonstructural (NS) protein 3/4A protease inhibitor that was identified jointly by AbbVie and Enanta Pharmaceuticals. It is being developed as a treatment of chronic hepatitis C infection in co-formulation with an HCV NS5A inhibitor pibrentasvir. Together they demonstrated potent antiviral activity against major HCV genotypes and high barriers to resistance in vitro.

Glecaprevir/pibrentasvir (G/P), sold under the brand names Mavyret and Maviret, is a fixed-dose combination medication used to treat hepatitis C. It contains glecaprevir and pibrentasvir. It works against all six types of hepatitis C. At twelve weeks following treatment between 81% and 100% of people have no evidence of hepatitis C. It is taken once a day by mouth with food.
Sofosbuvir/velpatasvir/voxilaprevir, sold under the brand name Vosevi, is a fixed-dose combination medication for the treatment of hepatitis C. It contains sofosbuvir, a hepatitis C virus (HCV) nucleotide analog NS5B polymerase inhibitor; velpatasvir, an HCV NS5A inhibitor; and voxilaprevir an HCV NS3/4A protease inhibitor.
Non-structural protein 5B (NS5B) inhibitors are a class of direct-acting antivirals widely used in the treatment of chronic hepatitis C. Depending on site of action and chemical composition, NS5B inhibitors may be categorized into three classes—nucleoside active site inhibitors (NIs), non-nucleoside allosteric inhibitors, and pyrophosphate analogues. Subsequently, all three classes are then subclassified. All inhibit RNA synthesis by NS5B but at different stages/sites resulting in inability of viral RNA replication. Expression of direct-acting NS5B inhibitors does not take place in cells that are not infected by hepatitis C virus, which seems to be beneficial for this class of drugs.