
Nootropics, colloquially brain supplements, smart drugs and cognitive enhancers, are natural, semisynthetic or synthetic compounds which purportedly improve cognitive functions, such as executive functions, attention or memory.

CX717 is an ampakine compound created by Christopher Marrs and Gary Rogers in 1996 at Cortex Pharmaceuticals. It affects the neurotransmitter glutamate, with trials showing the drug improves cognitive functioning and memory.
Racetams are a class of drugs that share a pyrrolidone nucleus. Some, such as piracetam, aniracetam, oxiracetam, pramiracetam and phenylpiracetam are considered nootropics. Others such as levetiracetam, brivaracetam, and seletracetam are anticonvulsants.

The 5HT6 receptor is a subtype of 5HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gs and mediates excitatory neurotransmission. HTR6 denotes the human gene encoding for the receptor.
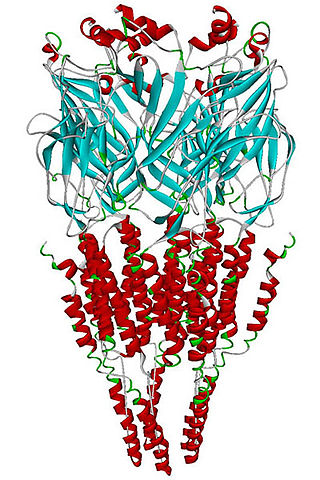
The alpha-7 nicotinic receptor, also known as the α7 receptor, is a type of nicotinic acetylcholine receptor implicated in long-term memory, consisting entirely of α7 subunits. As with other nicotinic acetylcholine receptors, functional α7 receptors are pentameric [i.e., (α7)5 stoichiometry].

IDRA-21 is a positive allosteric modulator of the AMPA receptor and a benzothiadiazine derivative. It is a chiral molecule, with (+)-IDRA-21 being the active form.

ABT-418 is a drug developed by Abbott, that has nootropic, neuroprotective and anxiolytic effects, and has been researched for treatment of both Alzheimer's disease and ADHD. It acts as an agonist at neural nicotinic acetylcholine receptors, subtype-selective binding with high affinity to the α4β2, α7/5-HT3, and α2β2 nicotinic acetylcholine receptors but not α3β4 receptors ABT-418 was reasonably effective for both applications and fairly well tolerated, but produced some side effects, principally nausea, and it is unclear whether ABT-418 itself will proceed to clinical development or if another similar drug will be used instead.
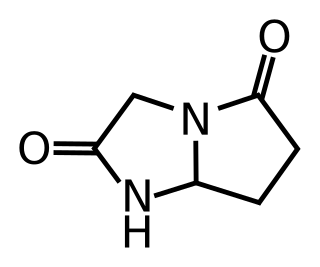
Dimiracetam is a nootropic drug of the racetam family, derivatives of which may have application in the treatment of neuropathic pain.

SB-357134 is a drug which is used in scientific research. It acts as a potent, selective and orally active 5-HT6 receptor antagonist. SB-357134 and other 5-HT6 antagonists show nootropic effects in animal studies, and have been proposed as potential novel treatments for cognitive disorders such as schizophrenia and Alzheimer's disease.

Salvinorin B methoxymethyl ether is a semi-synthetic analogue of the natural product salvinorin A used in scientific research. It has a longer duration of action of around 2–3 hours, compared to less than 30 minutes for salvinorin A, and has increased affinity and potency at the κ-opioid receptor. It is prepared from salvinorin B. The crystal structure is almost superimposable with that of salvinorin A. Structures bound to the κ-opioid receptor have also been reported.

N6-Cyclopentyladenosine (CPA) is a drug which acts as a selective adenosine A1 receptor agonist. It has mainly cardiovascular effects with only subtle alterations of behavior. CPA is widely used in scientific research into the adenosine receptors and has been used to derive a large family of derivatives.

Sunifiram is an experimental drug which has antiamnesic effects in animal studies and with significantly higher potency than piracetam. Sunifiram is a molecular simplification of unifiram (DM-232). Another analogue is sapunifiram (MN-19). As of 2016, sunifiram had not been subjected to toxicology testing, nor to any human clinical trials, and is not approved for use anywhere in the world.

PNU-282,987 is a drug that acts as a potent and selective agonist for the α7 subtype of neural nicotinic acetylcholine receptors. In animal studies, it shows nootropic effects, and derivatives may be useful in the treatment of schizophrenia, although PNU-282,987 is not suitable for use in humans because of excessive inhibition of the hERG antitarget. PNU-282987 has been shown to initiate signaling that leads to adult neurogeneis in mammals.

A-366,833 is a drug developed by Abbott, which acts as an agonist at neural nicotinic acetylcholine receptors selective for the α4β2 subtype, and has been researched for use as an analgesic, although it has not passed clinical trials. Its structure has a nicotinonitrile (3-cyanopyridine) core bound through C5 to the N6 of (1R,5S)-3,6-diazabicyclo[3.2.0]heptane.

TC-2216 is a drug developed by Targacept which acts as a partial agonist at neural nicotinic acetylcholine receptors and was researched for the treatment of anxiety and depression. It was unsuccessful as a therapeutic but is still used in pharmacological research as an alpha4beta2-selective antagonist.

TC-1698 is a drug developed by Targacept which acts as a partial agonist for the α7 subtype of neural nicotinic acetylcholine receptors. It has neuroprotective effects in animal studies, and has been used as a lead compound to find further potent derivatives.
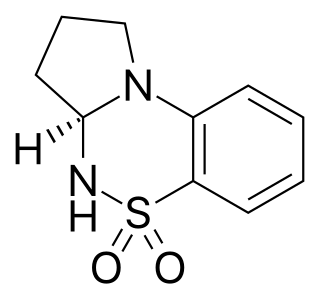
S-18986 is a positive allosteric modulator of the AMPA receptor related to cyclothiazide. It has nootropic and neuroprotective effects in animal studies, and induces both production of BDNF and AMPA-mediated release of noradrenaline and acetylcholine in the hippocampus and frontal cortex of the brain.

PD-102,807 is a drug which acts as a selective antagonist for the muscarinic acetylcholine receptor M4. It is used in scientific research for studying the effects of the different muscarinic receptor subtypes in the body and brain.
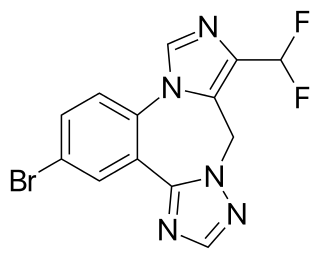
Ro4938581 is a nootropic drug invented in 2009 by a team working for Hoffmann-La Roche, which acts as a subtype-selective inverse agonist at the α5 subtype of the benzodiazepine binding site on the GABAA receptor. It has good selectivity for the α5 subtype and did not produce convulsant or anxiogenic effects in animal studies, making it a promising potential nootropic. Ro4938581 and a related derivative basmisanil have subsequently been investigated for the alleviation of cognitive dysfunction in Down syndrome.
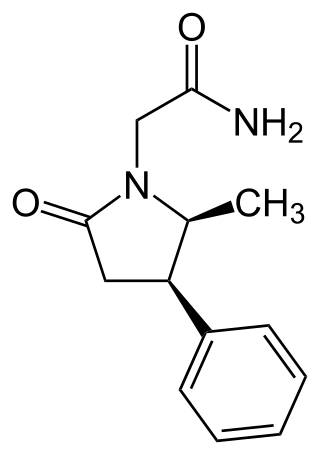
Methylphenylpiracetam is a derivative of piracetam and a positive allosteric modulator of the sigma-1 receptor. It differs from phenylpiracetam by having a methyl group.





















