 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Breinox, Dinagen, Lucetam, Nootropil, Nootropyl, Oikamid, Piracetam, others |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth, parenteral, inhalation |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Onset of action | Swiftly following administration. Food delays time to peak concentration by 1.5 h approximately to 2–3 h since dosing. [2] |
| Elimination half-life | 4–5 hours |
| Excretion | Urinary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.028.466 |
| Chemical and physical data | |
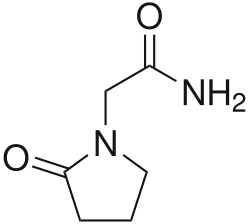
| Formula | C6H10N2O2 |
| Molar mass | 142.158 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 152 °C (306 °F) |
| |
| |
| (verify) | |
Piracetam is a drug that has efficacy in cognitive disorders, vertigo, cortical myoclonus, dyslexia, and sickle cell anemia; sources differ on its usefulness for dementia. [3] [4] [5] Piracetam is sold as a medication in many European countries. Piracetam in the United States is not approved for general use. [6]
Contents
- Efficacy
- Dementia
- Depression and anxiety
- Attention deficit hyperactivity disorder (ADHD)
- Other
- Anti-vasospasm
- Side effects
- Toxicity
- Mechanisms of action
- History
- Society and culture
- Legal status
- Regulatory status
- See also
- References
- External links
Piracetam is in the racetams group, with chemical name 2-oxo-1-pyrrolidine acetamide. It is a cyclic derivative of the neurotransmitter GABA [4] and shares the same 2-oxo-pyrrolidone base structure with pyroglutamic acid. Related drugs include the anticonvulsants levetiracetam and brivaracetam, and the putative nootropics aniracetam and phenylpiracetam.