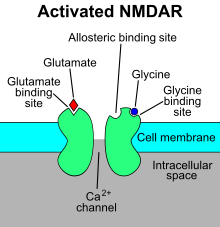
The N-methyl-D-aspartatereceptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA and kainate receptors. Depending on its subunit composition, its ligands are glutamate and glycine (or D-serine). However, the binding of the ligands is typically not sufficient to open the channel as it may be blocked by Mg2+ ions which are only removed when the neuron is sufficiently depolarized. Thus, the channel acts as a “coincidence detector” and only once both of these conditions are met, the channel opens and it allows positively charged ions (cations) to flow through the cell membrane. The NMDA receptor is thought to be very important for controlling synaptic plasticity and mediating learning and memory functions.

Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in response to the binding of a chemical messenger (i.e. a ligand), such as a neurotransmitter.

NMDA receptor antagonists are a class of drugs that work to antagonize, or inhibit the action of, the N-Methyl-D-aspartate receptor (NMDAR). They are commonly used as anesthetics for human and non-human animals; the state of anesthesia they induce is referred to as dissociative anesthesia.

Allopregnanolone is a naturally occurring neurosteroid which is made in the body from the hormone progesterone. As a medication, allopregnanolone is referred to as brexanolone, sold under the brand name Zulresso, and used to treat postpartum depression. It is given by injection into a vein.

Glutamate [NMDA] receptor subunit epsilon-2, also known as N-methyl D-aspartate receptor subtype 2B, is a protein that in humans is encoded by the GRIN2B gene.

Selfotel (CGS-19755) is a drug which acts as a competitive NMDA antagonist, directly competing with glutamate for binding to the receptor. Initial studies showed it to have anticonvulsant, anxiolytic, analgesic and neuroprotective effects, and it was originally researched for the treatment of stroke, but subsequent animal and human studies showed phencyclidine-like effects, as well as limited efficacy and evidence for possible neurotoxicity under some conditions, and so clinical development was ultimately discontinued.

Traxoprodil is a drug developed by Pfizer which acts as an NMDA antagonist, selective for the NR2B subunit. It has neuroprotective, analgesic, and anti-Parkinsonian effects in animal studies. Traxoprodil has been researched in humans as a potential treatment to lessen the damage to the brain after stroke, but results from clinical trials showed only modest benefit. The drug was found to cause EKG abnormalities and its clinical development was stopped. More recent animal studies have suggested traxoprodil may exhibit rapid-acting antidepressant effects similar to those of ketamine, although there is some evidence for similar psychoactive side effects and abuse potential at higher doses, which might limit clinical acceptance of traxoprodil for this application.

Rapastinel is a novel antidepressant that was under development by Allergan as an adjunctive therapy for the treatment of treatment-resistant depression. It is a centrally active, intravenously administered amidated tetrapeptide that acts as a novel and selective modulator of the NMDA receptor. The drug is a rapid-acting and long-lasting antidepressant as well as robust cognitive enhancer by virtue of its ability to enhance NMDA receptor-mediated signal transduction and synaptic plasticity.

Lanicemine (AZD6765) is a low-trapping NMDA receptor antagonist that was under development by AstraZeneca for the management of severe and treatment-resistant depression. Lanicemine differs from ketamine in that it is a low-trapping NMDA receptor antagonist, showing similar rapid-acting antidepressant effects to ketamine in clinical trials but with little or no psychotomimetic side effects. However, lanicemine did not meet study endpoints, and its development was terminated by AstraZeneca in 2013.

7-Chlorokynurenic acid (7-CKA) is a tool compound that acts as a potent and selective competitive antagonist of the glycine site of the NMDA receptor. It produces ketamine-like rapid antidepressant effects in animal models of depression. However, 7-CKA is unable to cross the blood-brain-barrier, and for this reason, is unsuitable for clinical use. As a result, a centrally-penetrant prodrug of 7-CKA, 4-chlorokynurenine (AV-101), has been developed for use in humans, and is being studied in clinical trials as a potential treatment for major depressive disorder, and anti-nociception. In addition to antagonizing the NMDA receptor, 7-CKA also acts as a potent inhibitor of the reuptake of glutamate into synaptic vesicles, an action that it mediates via competitive blockade of vesicular glutamate transporters.
The pharmacology of antidepressants is not entirely clear. The earliest and probably most widely accepted scientific theory of antidepressant action is the monoamine hypothesis, which states that depression is due to an imbalance of the monoamine neurotransmitters. It was originally proposed based on the observation that certain hydrazine anti-tuberculosis agents produce antidepressant effects, which was later linked to their inhibitory effects on monoamine oxidase, the enzyme that catalyses the breakdown of the monoamine neurotransmitters. All currently marketed antidepressants have the monoamine hypothesis as their theoretical basis, with the possible exception of agomelatine which acts on a dual melatonergic-serotonergic pathway. Despite the success of the monoamine hypothesis it has a number of limitations: for one, all monoaminergic antidepressants have a delayed onset of action of at least a week; and secondly, there are a sizeable portion (>40%) of depressed patients that do not adequately respond to monoaminergic antidepressants. Further evidence to the contrary of the monoamine hypothesis are the recent findings that a single intravenous infusion with ketamine, an antagonist of the NMDA receptor — a type of glutamate receptor — produces rapid, robust and sustained antidepressant effects. Monoamine precursor depletion also fails to alter mood. To overcome these flaws with the monoamine hypothesis a number of alternative hypotheses have been proposed, including the glutamate, neurogenic, epigenetic, cortisol hypersecretion and inflammatory hypotheses. Another hypothesis that has been proposed which would explain the delay is the hypothesis that monoamines don't directly influence mood, but influence emotional perception biases.

Apimostinel is an investigational antidepressant, acting as a novel and selective modulator of the NMDA receptor. It is currently under development for the acute treatment of major depressive disorder (MDD) by Gate Neurosciences, and previously by Naurex and Allergan. As of February 2015, an intravenous formulation of apimostinel has completed a phase IIa clinical trial for MDD.
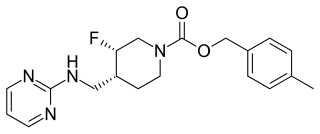
Rislenemdaz is an orally active, selective NMDA receptor subunit 2B (NR2B) antagonist which is under development by Cerecor in the United States as an adjunctive therapy for treatment-resistant depression (TRD). In November 2013, phase II clinical trials were initiated, and in the same month, rislenemdaz received Fast Track Designation from the Food and Drug Administration for TRD.

Arketamine (developmental code names PCN-101, HR-071603), also known as (R)-ketamine or (R)-(−)-ketamine, is the (R)-(−) enantiomer of ketamine. Similarly to racemic ketamine and esketamine, the S(+) enantiomer of ketamine, arketamine is biologically active; however, it is less potent as an NMDA receptor antagonist and anesthetic and thus has never been approved or marketed for clinical use as an enantiopure drug. Arketamine is currently in clinical development as a novel antidepressant.

Hydroxynorketamine (HNK), or 6-hydroxynorketamine, is a minor metabolite of the anesthetic, dissociative, and antidepressant drug ketamine. It is formed by hydroxylation of the intermediate norketamine, another metabolite of ketamine. As of late 2019, (2R,6R)-HNK is in clinical trials for the treatment of depression.
Cycloserine/lurasidone, developmental code name NRX-101 and tentative brand name Cyclurad, is a combination formulation of the antibiotic D-cycloserine, an antagonist of the glycine site of the NMDA receptor, and lurasidone, an atypical antipsychotic, which is under development by NeuroRx for the treatment of acute suicidal ideation/behavior (ASIB). As of May 2016, it has completed a phase II clinical trial for bipolar depression. In September 2017, the drug received fast track designation for bipolar depression from the United States Food and Drug Administration.
Immuno-psychiatry, according to Pariante, is a discipline that studies the connection between the brain and the immune system. It differs from psychoneuroimmunology by postulating that behaviors and emotions are governed by peripheral immune mechanisms. Depression, for instance, is seen as malfunctioning of the immune system.

Dextromethorphan/bupropion (DXM/BUP), sold under the brand name Auvelity, is a combination medication for the treatment of major depressive disorder (MDD). Its active components are dextromethorphan (DXM) and bupropion. Patients who stayed on the medication had an average of 11% greater reduction in depressive symptoms than placebo in an FDA approval trial. It is taken as a tablet by mouth.
Psychoplastogens are a group of small molecule drugs that produce rapid and sustained effects on neuronal structure and function, intended to manifest therapeutic benefit after a single administration. Several existing psychoplastogens have been identified and their therapeutic effects demonstrated; several are presently at various stages of development as medications including Ketamine, MDMA, Scopolamine, and the serotonergic psychedelics, including LSD, psilocin, DMT, and 5-MeO-DMT. Compounds of this sort are being explored as therapeutics for a variety of brain disorders including depression, addiction, and PTSD. The ability to rapidly promote neuronal changes via mechanisms of neuroplasticity was recently discovered as the common therapeutic activity and mechanism of action.