Ethyl loflazepate is a drug which is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. In animal studies it was found to have low toxicity, although in rats evidence of pulmonary phospholipidosis occurred with pulmonary foam cells developing with long-term use of very high doses. Its elimination half-life is 51–103 hours. Its mechanism of action is similar to other benzodiazepines. Ethyl loflazepate also produces an active metabolite which is stronger than the parent compound. Ethyl loflazepate was designed to be a prodrug for descarboxyloflazepate, its active metabolite. It is the active metabolite which is responsible for most of the pharmacological effects rather than ethyl loflazepate. The main metabolites of ethyl loflazepate are descarbethoxyloflazepate, loflazepate and 3-hydroxydescarbethoxyloflazepate. Accumulation of the active metabolites of ethyl loflazepate are not affected by those with kidney failure or impairment. The symptoms of an overdose of ethyl loflazepate include sleepiness, agitation and ataxia. Hypotonia may also occur in severe cases. These symptoms occur much more frequently and severely in children. Death from therapeutic maintenance doses of ethyl loflazepate taken for 2 – 3 weeks has been reported in 3 elderly patients. The cause of death was asphyxia due to benzodiazepine toxicity. High doses of the antidepressant fluvoxamine may potentiate the adverse effects of ethyl loflazepate.

Fludroxycortide, also known as flurandrenolide (USAN) and flurandrenolone, is a synthetic topical corticosteroid and is used as an anti-inflammatory treatment for use on skin irritations. Trade names include Haelan and Cordran.

Oxatomide, sold under the brand name Tinset among others, is a antihistamine of the diphenylmethylpiperazine family which is marketed in Europe, Japan, and a number of other countries. It was discovered at Janssen Pharmaceutica in 1975. Oxatomide lacks any anticholinergic effects. In addition to its H1 receptor antagonism, it also possesses antiserotonergic activity similarly to hydroxyzine.
Mazaticol (Pentona) is an anticholinergic used as an antiparkinsonian agent in Japan.

Setiptiline, also known as teciptiline, is a tetracyclic antidepressant (TeCA) that acts as a noradrenergic and specific serotonergic antidepressant (NaSSA). It was launched in 1989 for the treatment of depression in Japan by Mochida.
Flutoprazepam (Restas) is a drug which is a benzodiazepine. It was patented in Japan by Sumitomo in 1972 and its medical use remains mostly confined to that country. Its muscle relaxant properties are approximately equivalent to those of diazepam - however, it has more powerful sedative, hypnotic, anxiolytic and anticonvulsant effects and is around four times more potent by weight compared to diazepam. It is longer acting than diazepam due to its long-acting active metabolites, which contribute significantly to its effects. Its principal active metabolite is n-desalkylflurazepam, also known as norflurazepam, which is also a principal metabolite of flurazepam.

Rilmazafone is a water-soluble prodrug developed in Japan. Inside the human body, rilmazafone is converted into several benzodiazepine metabolites that have sedative and hypnotic effects.
Lafutidine (INN) is a second generation histamine H2 receptor antagonist having multimodal mechanism of action and used to treat gastrointestinal disorders. It is marketed in South Korea, Japan and India.

Taltirelin is a thyrotropin-releasing hormone (TRH) analog, which mimics the physiological actions of TRH, but with a much longer half-life and duration of effects, and little development of tolerance following prolonged dosing. It has nootropic, neuroprotective and analgesic effects.
Mofezolac (INN), sold under the name Disopain in Japan, is a nonsteroidal anti-inflammatory drug (NSAID) used for its analgesic and anti-inflammatory actions. It is often prescribed for rheumatoid arthritis, lower back pain, frozen shoulder, and pain management after surgery or trauma. It is also being investigated for potential use in the treatment of neuroinflammation.
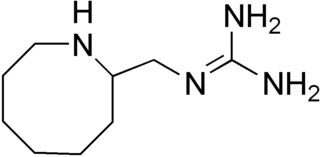
Guanazodine is a hypotensive sympatholytic drug.

Mosapramine (Cremin) is an atypical antipsychotic used in Japan for the treatment of schizophrenia. It is a potent dopamine antagonist with high affinity to the D2, D3, and D4 receptors, and with moderate affinity for the 5-HT2 receptors.
Acotiamide, sold under the brand name Acofide, is a medication manufactured and approved in Japan for the treatment of postprandial fullness, upper abdominal bloating, and early satiation due to functional dyspepsia. It acts as an acetylcholinesterase inhibitor.

Phellodendrine is an alkaloid isolated originally from Phellodendron amurense (Rutaceae).
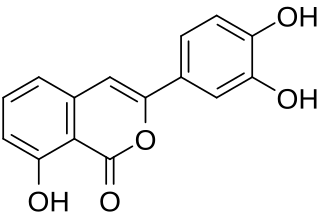
Thunberginol A is an isocoumarin found in Hydrangea macrophylla and the herbal preparation hydrangeae dulcis folium which is produced from its leaves.
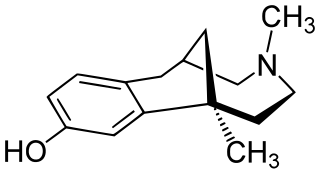
Eptazocine (Sedapain) is an opioid analgesic which was introduced in Japan by Morishita in 1987. It acts as a mixed κ-opioid receptor agonist and μ-opioid receptor antagonist.

Aceglutamide, or aceglutamide aluminium, also known as acetylglutamine, is a psychostimulant, nootropic, and antiulcer agent that is marketed in Spain and Japan. It is an acetylated form of the amino acid L-glutamine, the precursor of glutamate in the body and brain. Aceglutamide functions as a prodrug to glutamine with improved potency and stability.
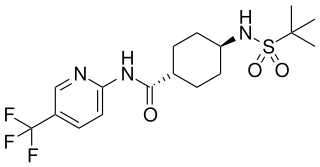
Velneperit (S-2367) is a drug developed by Shionogi, which acts as a potent and selective antagonist for the Neuropeptide Y receptor Y5. It has anorectic effects and was developed as a possible treatment for obesity, but was discontinued from further development after disappointing results in Phase II clinical trials. However it was still considered a successful proof of concept of the potential of Y5 receptor antagonists as possible anti-obesity agents in future.
Morpholine salicylate is a nonsteroidal anti-inflammatory drug.

Ro05-4082 is a benzodiazepine derivative developed in the 1970s. It has sedative and hypnotic properties, and has around the same potency as clonazepam itself. It was never introduced into clinical use. It is a structural isomer of meclonazepam (3-methylclonazepam), and similarly has been sold as a designer drug, first being identified in Sweden in 2017.