Antidepressants are a class of medications used to treat major depressive disorder, anxiety disorders, chronic pain, and addiction.
An anxiolytic is a medication or other intervention that reduces anxiety. This effect is in contrast to anxiogenic agents which increase anxiety. Anxiolytic medications are used for the treatment of anxiety disorders and their related psychological and physical symptoms.

Monoamine transporters (MATs) are proteins that function as integral plasma-membrane transporters to regulate concentrations of extracellular monoamine neurotransmitters. The three major classes are serotonin transporters (SERTs), dopamine transporters (DATs), and norepinephrine transporters (NETs) and are responsible for the reuptake of their associated amine neurotransmitters. MATs are located just outside the synaptic cleft (peri-synaptically), transporting monoamine transmitter overflow from the synaptic cleft back to the cytoplasm of the pre-synaptic neuron. MAT regulation generally occurs through protein phosphorylation and post-translational modification. Due to their significance in neuronal signaling, MATs are commonly associated with drugs used to treat mental disorders as well as recreational drugs. Compounds targeting MATs range from medications such as the wide variety of tricyclic antidepressants, selective serotonin reuptake inhibitors such as fluoxetine (Prozac) to stimulant medications such as methylphenidate (Ritalin) and amphetamine in its many forms and derivatives methamphetamine (Desoxyn) and lisdexamfetamine (Vyvanse). Furthermore, drugs such as MDMA and natural alkaloids such as cocaine exert their effects in part by their interaction with MATs, by blocking the transporters from mopping up dopamine, serotonin, and other neurotransmitters from the synapse.

Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant medications used to treat major depressive disorder (MDD), anxiety disorders, social phobia, chronic neuropathic pain, fibromyalgia syndrome (FMS), and menopausal symptoms. Off-label uses include treatments for attention-deficit hyperactivity disorder (ADHD), obsessive–compulsive disorder (OCD), and migraine prevention. SNRIs are monoamine reuptake inhibitors; specifically, they inhibit the reuptake of serotonin and norepinephrine. These neurotransmitters are thought to play an important role in mood regulation. SNRIs can be contrasted with the selective serotonin reuptake inhibitors (SSRIs) and norepinephrine reuptake inhibitors (NRIs), which act upon single neurotransmitters.

A norepinephrine reuptake inhibitor or noradrenaline reuptake inhibitor or adrenergic reuptake inhibitor (ARI), is a type of drug that acts as a reuptake inhibitor for the neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline) by blocking the action of the norepinephrine transporter (NET). This in turn leads to increased extracellular concentrations of norepinephrine and epinephrine and therefore can increase adrenergic neurotransmission.

Tetrabenazine is a drug for the symptomatic treatment of hyperkinetic movement disorders. It is sold under the brand names Nitoman and Xenazine among others. On August 15, 2008, the U.S. Food and Drug Administration approved the use of tetrabenazine to treat chorea associated with Huntington's disease. Although other drugs had been used "off label," tetrabenazine was the first approved treatment for Huntington's disease in the U.S. The compound has been known since the 1950s.

The norepinephrine transporter (NET), also known as noradrenaline transporter (NAT), is a protein that in humans is encoded by the solute carrier family 6 member 2 (SLC6A2) gene.

Nomifensine, sold under the brand names Merital and Alival, is a norepinephrine–dopamine reuptake inhibitor (NDRI), i.e. a drug that increases the amount of synaptic norepinephrine and dopamine available to receptors by blocking the dopamine and norepinephrine reuptake transporters. This is a mechanism of action shared by some recreational drugs like cocaine and the medication tametraline (see DRI). Research showed that the (S)-isomer is responsible for activity.
A serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), also known as a triple reuptake inhibitor (TRI), is a type of drug that acts as a combined reuptake inhibitor of the monoamine neurotransmitters serotonin, norepinephrine, and dopamine. It does this by concomitantly inhibiting the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT), respectively. Inhibition of the reuptake of these neurotransmitters increases their extracellular concentrations and, therefore, results in an increase in serotonergic, adrenergic, and dopaminergic neurotransmission. The naturally-occurring and potent SNDRI cocaine is widely used recreationally and often illegally for the euphoric effects it produces.

Indatraline hydrochloride is an antidepressive agent and non-selective monoamine transporter inhibitor that blocks the reuptake of dopamine, norepinephrine, and serotonin with similar efficacy to cocaine. This compound may be used to treat cocaine addictions as its effects have a slower onset and a longer duration than those of cocaine. Lu 19-005 has been shown to block the action of methamphetamine and MDMA in laboratory experiments.

A serotonin reuptake inhibitor (SRI) is a type of drug which acts as a reuptake inhibitor of the neurotransmitter serotonin by blocking the action of the serotonin transporter (SERT). This in turn leads to increased extracellular concentrations of serotonin and, therefore, an increase in serotonergic neurotransmission. It is a type of monoamine reuptake inhibitor (MRI); other types of MRIs include dopamine reuptake inhibitors and norepinephrine reuptake inhibitors.

Nisoxetine, originally synthesized in the Lilly research laboratories during the early 1970s, is a potent and selective inhibitor for the reuptake of norepinephrine (noradrenaline) into synapses. It currently has no clinical applications in humans, although it was originally researched as an antidepressant. Nisoxetine is now widely used in scientific research as a standard selective norepinephrine reuptake inhibitor. It has been used to research obesity and energy balance, and exerts some local analgesia effects.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.

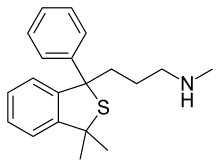
Talopram, also known as phthalapromine, is a selective norepinephrine reuptake inhibitor (NRI) which was researched for the management of depression in the 1960s and 1970s but was never commercialized. Along with talsupram, talopram is structurally related to the selective serotonin reuptake inhibitor (SSRI) citalopram, as well as to melitracen:
In 1971, the company hired Klaus Bøgesø as a medicinal chemist. Over the years Bøgesø turned out to have a Midas touch at the game of drug hunting, creating more molecules that made it to the market than almost any other medicinal chemist in the field. The challenge facing him in 1971 following his recruitment was to produce a selective norepinephrine reuptake inhibitor. Like other companies at the time, Lundbeck had little interest in an SSRI. Bøgesø began from an accident in the laboratory. Trying to create a derivative of their norepinephrine reuptake inhibiting antidepressant melitracen, Lundbeck chemists accidentally produced a new chemical — a phenylphthalene. Against all the odds, just like melitracen, this was also a selective norepinephrine reuptake inhibitor. Two potential antidepressants came out of this — talopram and tasulopram, which were pressed into clinical trials. Both however turned out to be energizing, and in a number of cases there were suicide attempts. The fact that there were suicide attempts appeared to confirm another proposal of Paul Kielholz, that activating antidepressants might lead to suicide. Lundbeck's experience suggested that norepinephrine reuptake inhibitors were likely to lead to just this problem. Lundbeck retreated, scared. If norepinephrine reuptake inhibitors were likely to trigger suicide, the greatest hazard of an antidepressant, then Kielholz's view suggested that an SSRI would be less likely to lead to suicide. Bøgesø's job was to see whether the new series of drugs could be converted into a series of SSRIs. Following a lead from Carlsson on how to do this, he converted talopram into citalopram, the most selective serotonin reuptake inhibitor to come to the market.

Tandamine is a selective norepinephrine reuptake inhibitor with a tricyclic structure. It was developed in the 1970s as an antidepressant but was never commercialized. Tandamine is analogous to pirandamine, which, instead, acts as a selective serotonin reuptake inhibitor (SSRI).

The plasma membrane monoamine transporter (PMAT) is a low-affinity monoamine transporter protein which in humans is encoded by the SLC29A4 gene. It is known alternatively as the human equilibrative nucleoside transporter-4 (hENT4). It was discovered in 2004 and has been identified as a potential alternate target for treating various conditions.
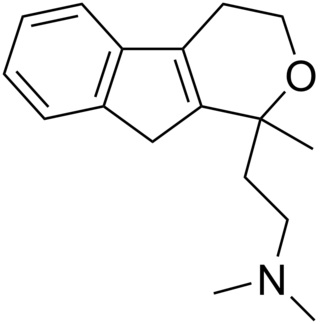
Pirandamine (AY-23,713) is a tricyclic derivative which acts as a selective serotonin reuptake inhibitor (SSRI). It was investigated in the 1970s as a potential antidepressant but clinical development was not commenced and it was never marketed. Pirandamine is structurally related to tandamine, which, in contrast, is a selective norepinephrine reuptake inhibitor.

Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs that are typically used as antidepressants in the treatment of major depressive disorder, anxiety disorders, and other psychological conditions.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.
Selective serotonin reuptake inhibitors, or serotonin-specific re-uptake inhibitor (SSRIs), are a class of chemical compounds that have application as antidepressants and in the treatment of depression and other psychiatric disorders. SSRIs are therapeutically useful in the treatment of panic disorder (PD), posttraumatic stress disorder (PTSD), social anxiety disorder, obsessive-compulsive disorder (OCD), premenstrual dysphoric disorder (PMDD), and anorexia. There is also clinical evidence of the value of SSRIs in the treatment of the symptoms of schizophrenia and their ability to prevent cardiovascular diseases.