
The substantia nigra (SN) is a basal ganglia structure located in the midbrain that plays an important role in reward and movement. Substantia nigra is Latin for "black substance", reflecting the fact that parts of the substantia nigra appear darker than neighboring areas due to high levels of neuromelanin in dopaminergic neurons. Parkinson's disease is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta.

MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is an organic compound. It is classified as a tetrahydropyridine. It is of interest as a precursor to the monoaminergic neurotoxin MPP+, which causes permanent symptoms of Parkinson's disease by destroying dopaminergic neurons in the substantia nigra of the brain. It has been used to study disease models in various animals.

Dopaminergic means "related to dopamine", a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain.

Entacapone, sold under the brand name Comtan among others, is a medication commonly used in combination with other medications for the treatment of Parkinson's disease. Entacapone together with levodopa and carbidopa allows levodopa to have a longer effect in the brain and reduces Parkinson's disease signs and symptoms for a greater length of time than levodopa and carbidopa therapy alone.
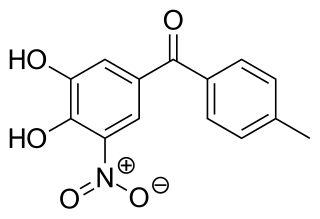
Tolcapone, sold under the brand name Tasmar, is a medication used to treat Parkinson's disease (PD). It is a selective, potent and reversible nitrocatechol-type inhibitor of the enzyme catechol-O-methyltransferase (COMT). It has demonstrated significant liver toxicity, which has led to suspension of marketing authorisations in a number of countries.

A dopamine agonist is a compound that activates dopamine receptors. There are two families of dopamine receptors, D1-like and D2-like. They are all G protein-coupled receptors. D1- and D5-receptors belong to the D1-like family and the D2-like family includes D2, D3 and D4 receptors. Dopamine agonists are primarily used in the treatment of the motor symptoms of Parkinson's disease, and to a lesser extent, in hyperprolactinemia and restless legs syndrome. They are also used off-label in the treatment of clinical depression. Impulse control disorders are associated with the use of dopamine agonists for whatever condition.
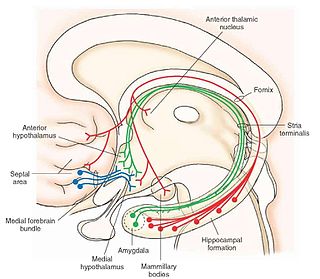
The medial forebrain bundle (MFB) is a neural pathway containing fibers from the basal olfactory regions, the periamygdaloid region and the septal nuclei, as well as fibers from brainstem regions, including the ventral tegmental area and nigrostriatal pathway.
In the management of Parkinson's disease, due to the chronic nature of Parkinson's disease (PD), a broad-based program is needed that includes patient and family education, support-group services, general wellness maintenance, exercise, and nutrition. At present, no cure for the disease is known, but medications or surgery can provide relief from the symptoms.

Dihydroergocryptine (DHEC), sold under the brand names Almirid and Cripar among others, is a dopamine agonist of the ergoline group that is used as an antiparkinson agent in the treatment of Parkinson's disease. It is taken by mouth.
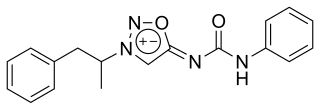
Mesocarb, sold under the brand name Sidnocarb or Sydnocarb and known by the developmental code name MLR-1017, is a psychostimulant medication which has been used in the treatment of psychiatric disorders and for a number of other indications in the Soviet Union and Russia. It is currently under development for the treatment of Parkinson's disease and sleep disorders. It is taken by mouth.

Bornaprine is a synthetic anticholinergic medication that is primarily used to treat Parkinson's disease. Additionally, bornaprine has been used to treat other disorders, including hyperhidrosis.

Tesofensine (NS2330) is a serotonin–noradrenaline–dopamine reuptake inhibitor from the phenyltropane family of drugs, which is being developed for the treatment of obesity. Tesofensine was originally developed by a Danish biotechnology company, NeuroSearch, who transferred the rights to Saniona in 2014.

(–)-Benzofuranylpropylaminopentane is an experimental drug related to selegiline which acts as a monoaminergic activity enhancer (MAE). It is orally active in animals.

Dimethocaine, also known as DMC or larocaine, is a compound with a stimulatory effect. This effect resembles that of cocaine, although dimethocaine appears to be less potent. Just like cocaine, dimethocaine is addictive due to its stimulation of the reward pathway in the brain. However, dimethocaine is a legal cocaine replacement in some countries and is even listed by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) under the category “synthetic cocaine derivatives”. The structure of dimethocaine, being a 4-aminobenzoic acid ester, resembles that of procaine. It is found as a white powder at room temperature.

Levodopa, also known as L-DOPA and sold under many brand names, is a dopaminergic medication which is used in the treatment of Parkinson's disease and certain other conditions like dopamine-responsive dystonia and restless legs syndrome. The drug is usually used and formulated in combination with a peripherally selective aromatic L-amino acid decarboxylase (AAAD) inhibitor like carbidopa or benserazide. Levodopa is taken by mouth, by inhalation, through an intestinal tube, or by administration into fat.

Remacemide is a drug which acts as a low-affinity NMDA antagonist with sodium channel blocking properties. It has been studied for the treatment of acute ischemic stroke, epilepsy, Huntington's disease, and Parkinson's disease.

Safinamide, sold under the brand name Xadago, is a medication used as treatment for Parkinson's disease with "off" episodes; it has multiple modes of action, including the inhibition of monoamine oxidase B.

BTS 74,398 is a centrally acting stimulant drug which was developed for the treatment of Parkinson's disease. It inhibits the synaptic reuptake of dopamine, serotonin and noradrenaline, making it a triple reuptake inhibitor. It was effective in animal models of Parkinson's disease, but was unsuccessful in human trials.

UWA-101 is a phenethylamine derivative researched as a potential treatment for Parkinson's disease. Its chemical structure is very similar to that of the illegal drug MDMA, the only difference being the replacement of the α-methyl group with an α-cyclopropyl group. MDMA has been found in animal studies and reported in unauthorised human self-experiments to be effective in the short-term relief of side-effects of Parkinson's disease therapy, most notably levodopa-induced dyskinesia. However the illegal status of MDMA and concerns about its potential for recreational use, neurotoxicity and potentially dangerous side effects mean that it is unlikely to be investigated for medical use in this application, and so alternative analogues were investigated.
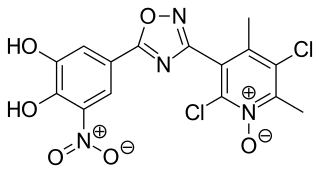
Opicapone, sold under the brand name Ongentys, is a medication which is administered together with levodopa in people with Parkinson's disease. Opicapone is a catechol-O-methyltransferase (COMT) inhibitor.



















