This article may require cleanup to meet Wikipedia's quality standards. The specific problem is: Unencyclopediac details in tables: compound-numbers specific to certain references ("7e", for example).(May 2019) |
Phenyltropanes (PTs) are a family of chemical compounds originally derived from structural modification of cocaine. The main feature differentiating phenyltropanes from cocaine is that they lack the ester functionality at the 3-position terminating in the benzene; thus, the phenyl is attached direct to the tropane skeleton (hence the name "phenyl"-tropane) with no further spacer that the cocaine benzoyloxy provided. The original purpose of phenyltropane-related research was to extirpate the cardiotoxicity inherent in the local anesthetic "numbing" capability of cocaine (which stems from the methylated benzoate ester being essential to cocaine's blockage of sodium channels, and which causes topical anesthesia) while retaining stimulant function. [a]
Contents
- 2-Carboxymethyl esters (phenyl-methylecgonines)
- (4′-Monosubstituted 2,3-Thiophene phenyl)-tropanes
- (3′,4′-Disubstituted phenyl)-tropanes
- (2′,4′-Disubstituted phenyl)-tropanes
- (3′,4′,5′-Trisubstituted para-methoxyphenyl)-tropanes
- (2′,4′,5′-Trisubstituted phenyl)-tropanes
- 2-Carbmethoxy modified (replaced/substituted)
- General 2-carbmethoxy modifications
- Carboxyaryl
- Carboxyalkyl
- Carboxamides
- Heterocycles
- Acyl (C2-propanoyl)
- Ester reduction
- 2-Alkane/Alkene
- Irreversible covalent (cf. ionic) C2 ligands
- Benztropine based (C2-position hetero-substituted) phenyltropanes
- F&B series (Biotin side-chains etc.)
- Miscellany (i.e. Misc./Miscellaneous) C2-substituents
- C2-truncated/descarboxyl (non-ecgonine w/o 2-position-replacement tropanes)
- Aryl-Tropenes
- Enantioselective nonstandard configurations (non-2β-,3β-)
- β,α Stereochemistry
- α,β Stereochemistry
- Arene equivalent alterations
- η6-3β-(transition metal complexed phenyl)tropanes
- 3-(2-thiophene) and 3-(2-furan)
- Thiophenyltropanes
- Diaryl
- 6/7-tropane position substituted
- 2β-carbomethoxy 6/7 substituted
- 3-butyl 6/7 substituted
- intermediate 6- & 7-position synthesis modified phenyltropanes
- 8-tropane (bridgehead) position modified
- Nortropanes (N-demethylated)
- N-replaced (S,O,C)
- N-alkyl
- Bridged N-constrained phenyltropanes (fused/tethered)
- Cycloalkane-ring alterations of the tropane ring system
- Azanonane (outer ring extended)
- Azabornane (outer ring contracted)
- Piperidine homologues (inner two-carbon bridge excised)
- Radiolabeled
- Transition metal complexes
- Select annotations of above
- Sister substances
- See also
- References
- Citations
- Im-pact indices (exact locations within sources cited) & foot-notations
- External links
Phenyltropane compounds present promising avenues of research into therapeutic applications, particularly in regard to addiction treatment. These compounds' uses vary depending on their construction and structure-activity relationship ranging from the treating of cocaine dependency to understanding the dopamine reward system in the human brain to treating Alzheimer's and Parkinson's diseases. (Since 2008 there have been continual additions to the list and enumerations of the plethora of types of chemicals that fall into the category of this substance profile. [2] ) Certain phenyltropanes can even be used as a smoking cessation aid (cf. RTI-29). Many of the compounds were first elucidated in published material by the Research Triangle Institute and are thus named with "RTI" serial-numbers (in this case the long form is either RTI-COC-n, for 'cocaine' "analog", or specifically RTI-4229-n of the subsequent numbers given below in this article) [b] Similarly, a number of others are named for Sterling-Winthrop pharmaceuticals ("WIN" serial-numbers) and Wake Forest University ("WF" serial-numbers). The following includes many of the phenyltropane class of drugs that have been made and studied.
- 3D rendering of troparil; which comprises a privileged scaffold of among the phenyltropane class of compounds.
- Troparil structure: cf. U.S. patent 5,496,953



























































































































































































































































































































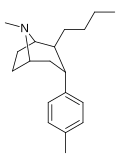




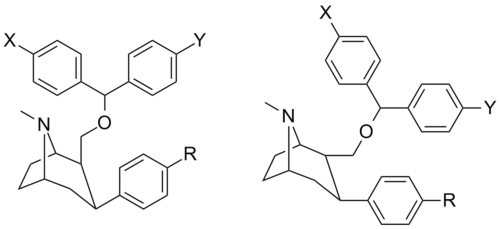




























































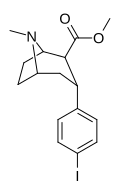

































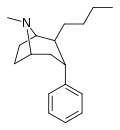









































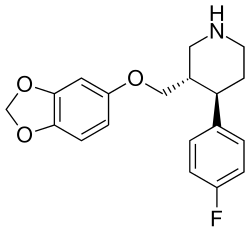












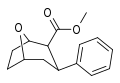






































































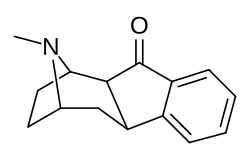

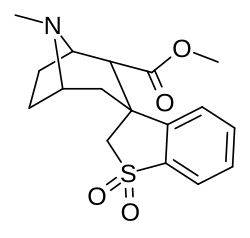






![2-exo-phenyl-7-azabicyclo[2.2.1]heptane:
The non-carboxylic (and DAT substrate, releasing agent) variant of exo-2-phenyl-7-azabicyclo(2.2.1)heptane-1-carboxylic acid (N.B. the carboxy in the latter shares the C1 tropane position with the two carbon nitrogen containing bridge; sharing in the leftmost (R) substitution of the above depiction & unlike the placement on the tropane for either the carbmethoxy or phenyl ring of the azabornane analogues given in this section)
With the carboxy ester function removed the resultant derived compound acts as a DAT substrate drug, thus an amphetaminergic releaser of MAT & VMAT, yet similar to phenyltropanes (that usually are only re-uptake ligands) cf. EXP-561 & BTQ. 2-exo-phenyl-7-azabicyclo(2-2-1)heptane.png](http://upload.wikimedia.org/wikipedia/commons/thumb/1/11/2-exo-phenyl-7-azabicyclo%282-2-1%29heptane.png/250px-2-exo-phenyl-7-azabicyclo%282-2-1%29heptane.png)










































