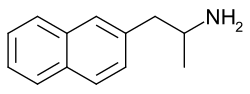

The substituted naphthylethylamines are a class of chemical compounds based on naphthalene. Many naphthylethylamines are naphthylaminopropanes (also known as naphthylisopropylamines) due to the presence of a methyl group at the alpha carbon of the alkyl chain. The naphthylethylamines are derivatives of the phenethylamines, while the naphthylaminopropanes are derivatives of the amphetamines.
Contents
There are two types of naphthylethylamines based on positional isomerism: 1-naphthylethylamines and 2-naphthylethylamines. Examples of these include 1-naphthylaminopropane (1-NAP) and 2-naphthylaminopropane (2-NAP), respectively.