
5-MeO-DET or 5-methoxy-N,N-diethyltryptamine is a hallucinogenic tryptamine.

WIN 35,428 is a stimulant drug used in scientific research. CFT is a phenyltropane based dopamine reuptake inhibitor and is structurally derived from cocaine. It is around 3-10x more potent than cocaine and lasts around 7 times longer based on animal studies. While the naphthalenedisulfonate salt is the most commonly used form in scientific research due to its high solubility in water, the free base and hydrochloride salts are known compounds and can also be produced. The tartrate is another salt form that is reported.
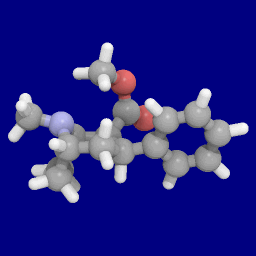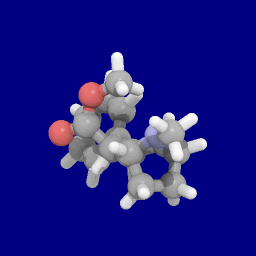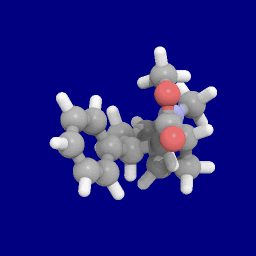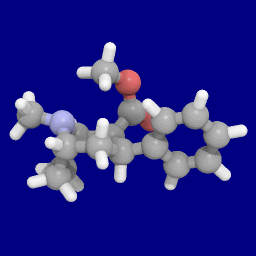
Phenyltropanes (PTs) were originally developed to reduce cocaine addiction and dependency. In general these compounds act as inhibitors of the plasmalemmal monoamine reuptake transporters. This research has spanned beyond the last couple decades, and has picked up its pace in recent times, creating numerous phenyltropanes as research into cocaine analogues garners interest to treat addiction.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine lacks the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.

Troparil is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.
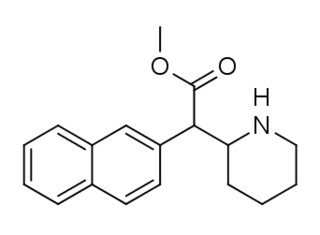
HDMP-28 or methylnaphthidate is a piperidine based stimulant drug, closely related to methylphenidate, but with the benzene ring replaced by naphthalene. It is a potent dopamine reuptake inhibitor, with several times the potency of methylphenidate and a short duration of action, and is a structural isomer of another potent dopamine reuptake inhibitor, N,O-Dimethyl-4-(2-naphthyl)piperidine-3-carboxylate. It has been sold as a designer drug since around 2015.

2-Benzylpiperidine is a stimulant drug of the arylpiperidine family. It is similar in structure to certain other stimulants such as methylphenidate and desoxypipradrol. However, it is far less potent as a monoamine reuptake inhibitor in comparison. The drug is little used as a stimulant, with its main use being as a synthetic intermediate in the manufacture of other drugs.
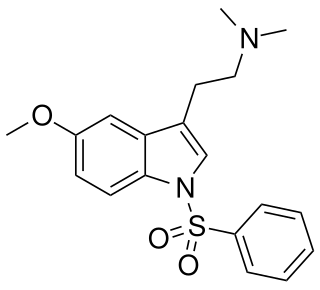
MS-245 is a tryptamine derivative used in scientific research. It acts as a selective 5-HT6 receptor antagonist with a Ki of 2.3 nM, and was derived through structure-activity relationship development of the selective 5-HT6 agonist EMDT. It has been used as a lead compound for further development of tryptamine-derived 5-HT6 antagonists. In animal studies it has been shown to boost the activity of, but not substitute for, both amphetamine and nicotine.

AMDA (9-Aminomethyl-9,10-dihydroanthracene) is an organic compound which acts as a potent and selective antagonist for the 5-HT2A receptor. It has been used to help study the shape of the 5-HT2A protein, and develop a large family of related derivatives with even higher potency and selectivity.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.
Substituted cathinones, or simply cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions. Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug.
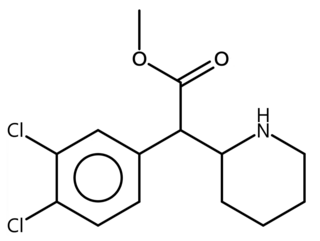
3,4-dichloromethylphenidate is a potent stimulant drug from the phenidate class closely related to methylphenidate. It acts as a potent serotonin-norepinephrine-dopamine reuptake inhibitor with a long duration of action. It has been sold online as a designer drug.
threo-4-Methylmethylphenidate (4-MeTMP) is a stimulant drug related to methylphenidate. It is slightly less potent than methylphenidate and has relatively low efficacy at blocking dopamine reuptake despite its high binding affinity, which led to its investigation as a possible substitute drug for treatment of stimulant abuse. On the other hand, several other simple ring-substituted derivatives of threo-methylphenidate such as the 4-fluoro and 3-chloro compounds are more potent than methylphenidate both in efficacy as dopamine reuptake inhibitors and in animal drug discrimination assays.

Metaphit is a research chemical that acts as an acylator of NMDARAn, sigma and DAT binding sites in the CNS. It is the m-isothiocyanate derivative of phencyclidine (PCP) and binds irreversibly to the PCP binding site on the NMDA receptor complex. However, later studies suggest the functionality of metaphit is mediated by sites not involved in PCP-induced passive avoidance deficit, and not related to the NMDA receptor complex. Metaphit was also shown to prevent d-amphetamine induced hyperactivity, while significantly depleting dopamine content in the nucleus accumbens. Metaphit was the first acylating ligand used to study the cocaine receptor. It is a structural isomer of the similar research compound fourphit, as it and metaphit both are isothiocyanate substituted derivatives of an analogous scaffold shared with PCP.

KM-233 is a synthetic cannabinoid drug which is a structural analog of Δ8-tetrahydrocannabinol (THC), the less active but more stable isomer of the active component of Cannabis. KM-233 differs from Δ8-THC by the pentyl side chain being replaced by a 1,1-dimethylbenzyl group. It has high binding affinity in vitro for both the CB1 and CB2 receptors, with a CB2 affinity of 0.91 nM and 13-fold selectivity over the CB1 receptor. In animal studies, it has been found to be a potential treatment for glioma, a form of brain tumor. Many related analogues are known where the 1,1-dimethylbenzyl group is substituted or replaced by other groups, with a fairly well established structure-activity relationship.

4-Methylcathinone (4-MC), also known as normephedrone is a stimulant drug of the cathinone group. It is an active metabolite of the better known drug mephedrone.

4-Fluoromethylphenidate is a stimulant drug that acts as a higher potency dopamine reuptake inhibitor than the closely related methylphenidate.

















