
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH. Initially thought essential only for infants, it has now been shown in longer-term studies to be essential for adults also. It is encoded by the codons CAU and CAC.

Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms in meta-substitution.

Camazepam is a benzodiazepine psychoactive drug, marketed under the brand names Albego, Limpidon and Paxor. It is the dimethyl carbamate ester of temazepam, a metabolite of diazepam. While it possesses anxiolytic, anticonvulsant, skeletal muscle relaxant and hypnotic properties it differs from other benzodiazepines in that its anxiolytic properties are particularly prominent but has comparatively limited anticonvulsant, hypnotic and skeletal muscle relaxant properties.

Naphthylaminopropane (PAL-287) is an experimental drug under investigation as of 2007 for the treatment of alcohol and stimulant addiction.

A GABA receptor agonist is a drug that is an agonist for one or more of the GABA receptors, producing typically sedative effects, and may also cause other effects such as anxiolytic, anticonvulsant, and muscle relaxant effects. There are three receptors of the gamma-aminobutyric acid. The two receptors GABA-α and GABA-ρ are ion channels that are permeable to chloride ions which reduces neuronal excitability. The GABA-β receptor belongs to the class of G-Protein coupled receptors that inhibit adenylyl cyclase, therefore leading to decreased cyclic adenosine monophosphate (cAMP). GABA-α and GABA-ρ receptors produce sedative and hypnotic effects and have anti-convulsion properties. GABA-β receptors also produce sedative effects. Furthermore, they lead to changes in gene transcription.

QH-II-66 (QH-ii-066) is a sedative drug which is a benzodiazepine derivative. It produces some of the same effects as other benzodiazepines, but is much more selective than most other drugs of this class and so produces somewhat less sedation and ataxia than other related drugs such as diazepam and triazolam, although it still retains anticonvulsant effects.

γ-Amino-β-hydroxybutyric acid (GABOB), also known as β-hydroxy-γ-aminobutyric acid (β-hydroxy-GABA), and sold under the brand name Gamibetal among others, is an anticonvulsant which is used for the treatment of epilepsy in Europe, Japan, and Mexico. It is a GABA analogue, or an analogue of the neurotransmitter γ-aminobutyric acid (GABA), and has been found to be an endogenous metabolite of GABA.

Dopamine beta-hydroxylase (DBH), also known as dopamine beta-monooxygenase, is an enzyme that in humans is encoded by the DBH gene. Dopamine beta-hydroxylase catalyzes the conversion of dopamine to norepinephrine.

In molecular biology, the protein domain SAICAR synthase is an enzyme which catalyses a reaction to create SAICAR. In enzymology, this enzyme is also known as phosphoribosylaminoimidazolesuccinocarboxamide synthase. It is an enzyme that catalyzes the chemical reaction
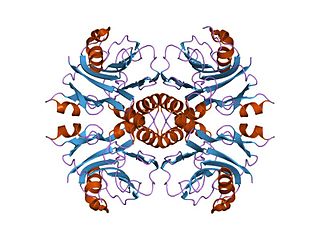
In enzymology, an IMP cyclohydrolase (EC 3.5.4.10) is an enzyme that catalyzes the chemical reaction
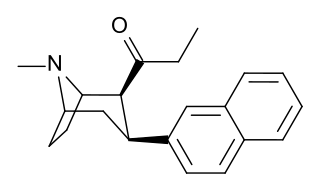
2β-Propanoyl-3β-(2-naphthyl)-tropane or WF-23 is a cocaine analogue. It is several hundred times more potent than cocaine at being a serotonin-norepinephrine-dopamine reuptake inhibitor.
Sodium channel blockers are drugs which impair the conduction of sodium ions (Na+) through sodium channels.

4-NEMD is a potent sedative drug which acts as a selective alpha-2 adrenergic agonist. It is closely related to dexmedetomidine but is several times more potent. Like other alpha-2 agonists, it produces sedative and muscle relaxant effects but without producing respiratory depression. It is not currently used in medicine but has been researched as the basis for a potential new generation of alpha-2 agonist drugs, which may have selectivity for the different subtypes of the alpha-2 receptor. It has two isomers, with the (S) isomer being the more potent, as with medetomidine. 4-NEMD was also investigated by the United States military as an anaesthetic agent, most likely for use in surgery but possibly also for use as a non-lethal incapacitating agent, although this has not been officially confirmed.

ZK-93426 (ethyl-5-isopropoxy-4-methyl-beta-carboline-3-carboxylate) is a drug from the beta-carboline family. It acts as a weak partial inverse agonist of benzodiazepine receptors, meaning that it causes the opposite effects to the benzodiazepine class of drugs and has anxiogenic properties, although unlike most benzodiazepine antagonists it is not a convulsant and actually has weak anticonvulsant effects. In human tests it produced alertness, restlessness and feelings of apprehension, and reversed the effect of the benzodiazepine lormetazepam. It was also shown to produce nootropic effects and increased release of acetylcholine.
These drugs are known in the UK as controlled drugs, because this is the term by which the act itself refers to them. In more general terms, however, many of these drugs are also controlled by the Medicines Act 1968, there are many other drugs which are controlled by the Medicines Act but not by the Misuse of Drugs Act, and some other drugs are controlled by other laws.

Naphyrone, also known as O-2482 and naphthylpyrovalerone, is a substituted cathinone drug derived from pyrovalerone that acts as a triple reuptake inhibitor, producing stimulant effects and has been reported as a novel designer drug. No safety or toxicity data is available on the drug.
Adimolol is antihypertensive agent which acts as a non-selective α1-, α2-, and β-adrenergic receptor antagonist.
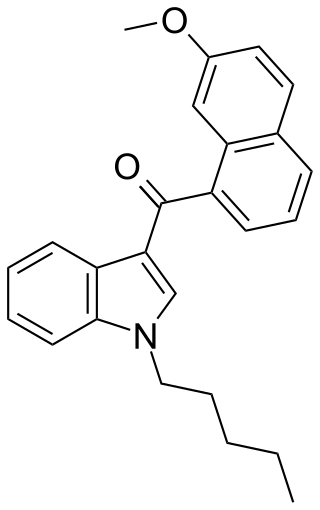
JWH-164 is a synthetic cannabinoid receptor agonist from the naphthoylindole family. It has approximately equal affinity for the CB1 and CB2 receptors, with a Ki of 6.6 nM at CB1 and 6.9 nM at CB2. JWH-164 is a positional isomer of the related compound JWH-081, but with a methoxy group at the 7-position of the naphthyl ring, rather than the 4-position as in JWH-081. Its potency is intermediate between that of JWH-081 and its ring unsubstituted derivative JWH-018, demonstrating that substitution of the naphthyl 7-position can also result in increased cannabinoid receptor binding affinity.
To combat the illicit synthetic cannabinoid industry many jurisdictions have created a system to control these cannabinoids through their general structure as opposed to their specific identity. In this way new analogs are already controlled before they are even created. A large number of cannabinoids have been grouped into classes based on similarities in their chemical structure, and these classes have been widely adopted across a variety of jurisdictions.
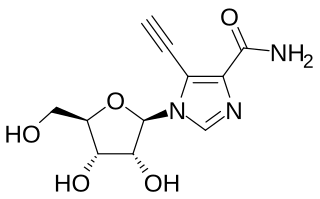
EICAR is a drug which acts as an inhibitor of the enzyme IMP dehydrogenase. It is a nucleoside derivative which has both anti-cancer and antiviral effects, and was originally developed for the treatment of leukemia, but was unsuccessful in human clinical trials. It has broad spectrum antiviral effects with activity against pox viruses, Semliki forest virus, Junin virus, reovirus, influenza, measles virus and respiratory syncytial virus among others, although it is not active against coronaviridae such as SARS-CoV-1. This useful spectrum of activity means that EICAR and related derivatives continue to be investigated for the treatment of viral diseases.



















