
5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

Azapirones are a class of drugs used as anxiolytics, antidepressants, and antipsychotics. They are commonly used as add-ons to other antidepressants, such as selective serotonin reuptake inhibitors (SSRIs).
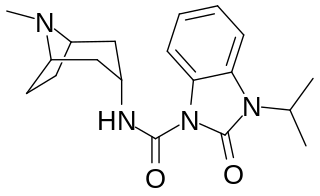
BIMU-8 is a drug which acts as a 5-HT4 receptor selective agonist. BIMU-8 was one of the first compounds of this class. The main action of BIMU-8 is to increase the rate of respiration by activating an area of the brain stem known as the pre-Botzinger complex.

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarisation and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

8-OH-DPAT is a research chemical of the aminotetralin chemical class which was developed in the 1980s and has been widely used to study the function of the 5-HT1A receptor. It was one of the first major 5-HT1A receptor full agonists to be discovered.
5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the HTR2B gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT).

Eglumetad is a research drug developed by Eli Lilly and Company, which is being investigated for its potential in the treatment of anxiety and drug addiction. It is a glutamate derived compound and its mode of action implies a novel mechanism.

WAY-100635 is a piperazine drug and research chemical widely used in scientific studies. It was originally believed to act as a selective 5-HT1A receptor antagonist, but subsequent research showed that it also acts as potent full agonist at the D4 receptor. It is sometimes referred to as a silent antagonist at the former receptor. It is closely related to WAY-100135.

Flesinoxan (DU-29,373) is a potent and selective 5-HT1A receptor partial/near-full agonist of the phenylpiperazine class. Originally developed as a potential antihypertensive drug, flesinoxan was later found to possess antidepressant and anxiolytic effects in animal tests. As a result, it was investigated in several small human pilot studies for the treatment of major depressive disorder, and was found to have robust effectiveness and very good tolerability. However, due to "management decisions", the development of flesinoxan was stopped and it was not pursued any further.

Zacopride is a potent antagonist at the 5-HT3 receptor and an agonist at the 5-HT4 receptor. It has anxiolytic and nootropic effects in animal models, with the (R)-(+)-enantiomer being the more active form. It also has antiemetic and pro-respiratory effects, both reducing sleep apnea and reversing opioid-induced respiratory depression in animal studies. Early animal trials have also revealed that administration of zacopride can reduce preference for and consumption of ethanol.

UH-232 ((+)-UH232) is a drug which acts as a subtype selective mixed agonist-antagonist for dopamine receptors, acting as a weak partial agonist at the D3 subtype, and an antagonist at D2Sh autoreceptors on dopaminergic nerve terminals. This causes dopamine release in the brain and has a stimulant effect, as well as blocking the behavioural effects of cocaine. It may also serve as a 5-HT2A receptor agonist, based on animal studies. It was investigated in clinical trials for the treatment of schizophrenia, but unexpectedly caused symptoms to become worse.
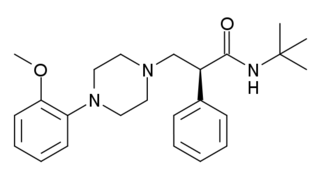
WAY-100135 is a serotonergic drug of the phenylpiperazine family which is used in scientific research. It acts as potent 5-HT1A receptor antagonist, and was originally believed to be highly selective, but further studies have demonstrated that it also acts as a partial agonist of the 5-HT1D receptor (pKi = 7.58; virtually the same affinity for 5-HT1A), and to a much lesser extent, of the 5-HT1B receptor (pKi = 5.82). These findings may have prompted the development of the related compound WAY-100635, another purportedly selective and even more potent 5-HT1A antagonist, which was synthesized shortly thereafter. However, WAY-100635 turned out to be non-selective as well, having been shown to act additionally as a potent D4 receptor agonist later on.
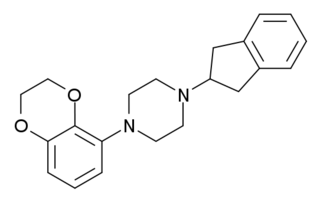
S-15535 is a phenylpiperazine drug which is a potent and highly selective 5-HT1A receptor ligand that acts as an agonist and antagonist at the presynaptic and postsynaptic 5-HT1A receptors, respectively. It has anxiolytic properties.

Eptapirone (F-11,440) is a very potent and highly selective 5-HT1A receptor full agonist of the azapirone family. Its affinity for the 5-HT1A receptor was reported to be 4.8 nM (Ki), and its intrinsic activity approximately equal to that of serotonin.
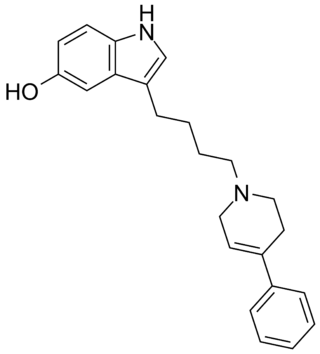
Roxindole (EMD-49,980) is a dopaminergic and serotonergic drug which was originally developed by Merck KGaA for the treatment of schizophrenia. In clinical trials its antipsychotic efficacy was only modest but it was unexpectedly found to produce potent and rapid antidepressant and anxiolytic effects. As a result, roxindole was further researched for the treatment of depression instead. It has also been investigated as a therapy for Parkinson's disease and prolactinoma.

Pruvanserin is a selective 5-HT2A receptor antagonist which was under development by Eli Lilly and Company for the treatment of insomnia. It was in phase II clinical trials in 2008 but appears to have been discontinued as it is no longer in the company's development pipeline. In addition to its sleep-improving properties, pruvanserin has also been shown to have antidepressant, anxiolytic, and working memory-enhancing effects in animal studies.
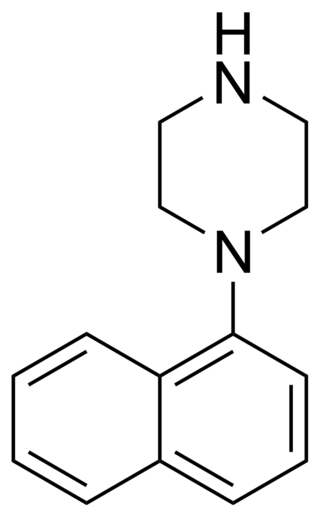
1-(1-Naphthyl)piperazine (1-NP) is a drug which is a phenylpiperazine derivative. It acts as a non-selective, mixed serotonergic agent, exerting partial agonism at the 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F receptors, while antagonizing the 5-HT2A, 5-HT2B, and 5-HT2C receptors. It has also been shown to possess high affinity for the 5-HT3, 5-HT5A, 5-HT6, and 5-HT7 receptors, and may bind to 5-HT4 and the SERT as well. In animals it produces effects including hyperphagia, hyperactivity, and anxiolysis, of which are all likely mediated predominantly or fully by blockade of the 5-HT2C receptor.

CSP-2503 is a potent and selective 5-HT1A receptor agonist, 5-HT2A receptor antagonist, and 5-HT3 receptor antagonist of the naphthylpiperazine class. First synthesized in 2003, it was designed based on computational models and QSAR studies. In rat studies, CSP-2503 has demonstrated anxiolytic effects, and thus has been suggested as a treatment for anxiety in humans with a multimodal mechanism of action.

SB-243213 is a research chemical which acts as a selective inverse agonist for the 5HT2C receptor and has anxiolytic effects. It has better than 100x selectivity for 5-HT2C over all other receptor subtypes tested, and a longer duration of action compared to older 5-HT2C antagonist ligands.
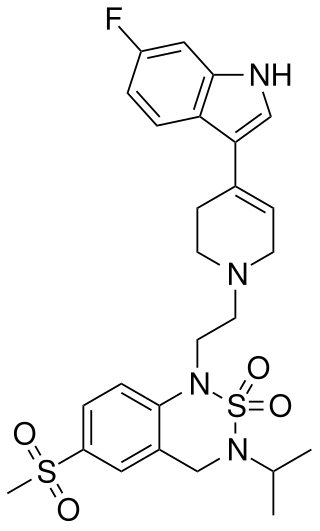
LY-393558 is a potent serotonin reuptake inhibitor and antagonist of the 5-HT1B, 5-HT1D, and 5-HT2A receptors. LY-393558 was also found to reduce serotonin-induced vasoconstriction, indicating that it may have therapeutic potential for the treatment of pulmonary hypertension.





















