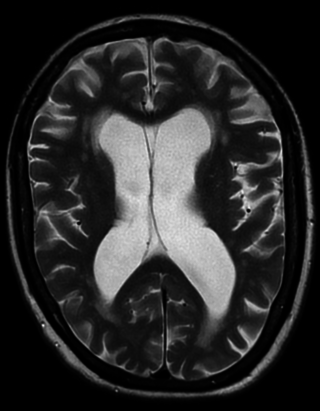
Vascular dementia is dementia caused by a series of strokes. Restricted blood flow due to strokes reduces oxygen and glucose delivery to the brain, causing cell injury and neurological deficits in the affected region. Subtypes of vascular dementia include subcortical vascular dementia, multi-infarct dementia, stroke-related dementia, and mixed dementia.

Cerebrovascular disease includes a variety of medical conditions that affect the blood vessels of the brain and the cerebral circulation. Arteries supplying oxygen and nutrients to the brain are often damaged or deformed in these disorders. The most common presentation of cerebrovascular disease is an ischemic stroke or mini-stroke and sometimes a hemorrhagic stroke. Hypertension is the most important contributing risk factor for stroke and cerebrovascular diseases as it can change the structure of blood vessels and result in atherosclerosis. Atherosclerosis narrows blood vessels in the brain, resulting in decreased cerebral perfusion. Other risk factors that contribute to stroke include smoking and diabetes. Narrowed cerebral arteries can lead to ischemic stroke, but continually elevated blood pressure can also cause tearing of vessels, leading to a hemorrhagic stroke.

Piracetam is a drug that has efficacy in cognitive disorders, vertigo, cortical myoclonus, dyslexia, and sickle cell anemia; sources differ on its usefulness for dementia. Piracetam is sold as a medication in many European countries. Sale of piracetam is not illegal in the United States, although it is not regulated nor approved by the FDA, so it is legally sold for research use only.

Ramipril, sold under the brand name Altace among others, is an ACE inhibitor type medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It can also be used as a preventative medication in patients over 55 years old to reduce the risk of having a heart attack, stroke or cardiovascular death in patients shown to be at high risk, such as some diabetics and patients with vascular disease. It is a reasonable initial treatment for high blood pressure. It is taken by mouth.
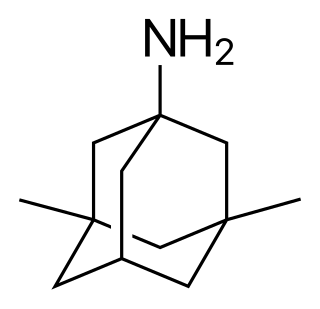
Memantine, sold under the brand name Namenda among others, is a medication used to slow the progression of moderate-to-severe Alzheimer's disease. It is taken by mouth.
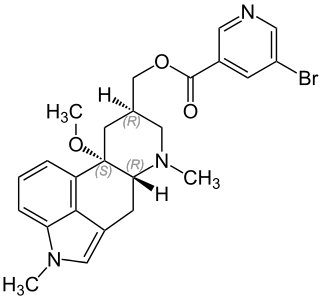
Nicergoline, sold under the brand name Sermion among others, is an ergot derivative used to treat senile dementia and other disorders with vascular origins. Internationally it has been used for frontotemporal dementia as well as early onset in Lewy body dementia and Parkinson's dementia. It decreases vascular resistance and increases arterial blood flow in the brain, improving the utilization of oxygen and glucose by brain cells. It has similar vasoactive properties in other areas of the body, particularly the lungs. Unlike many other ergolines, such as ergotamine, nicergoline is not associated with cardiac fibrosis.

Isoxsuprine is a drug used as a vasodilator in humans and equines. Isoxsuprine is a β2 adrenoreceptor agonist that causes direct relaxation of uterine and vascular smooth muscle via β2 receptors.

Tandospirone, sold under the brand name Sediel, is an anxiolytic and antidepressant medication used in Japan and China, where it is marketed by Dainippon Sumitomo Pharma. It is a member of the azapirone class of drugs and is closely related to other azapirones like buspirone and gepirone.
Benfluorex, sold under the brand name Mediator, is an anorectic and hypolipidemic agent that is structurally related to fenfluramine. It may improve glycemic control and decrease insulin resistance in people with poorly controlled type-2 diabetes.

Oxyfedrine, sold under the brand names Ildamen and Myofedrin among others, is a sympathomimetic agent and coronary vasodilator which is used in the treatment of coronary heart disease, angina pectoris, and acute myocardial infarction. It is taken by mouth or intravenously.
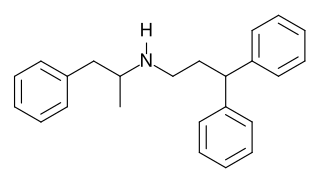
Prenylamine (Segontin) is a calcium channel blocker of the amphetamine chemical class that was used as a vasodilator in the treatment of angina pectoris.

Flupirtine is an aminopyridine that functions as a centrally acting non-opioid analgesic that was originally used as an analgesic for acute and chronic pain but in 2013 due to issues with liver toxicity, the European Medicines Agency restricted its use to acute pain, for no more than two weeks, and only for people who cannot use other painkillers. In March 2018, marketing authorisations for flupirtine were withdrawn following a European Medicines Agency recommendation based on the finding that the restrictions introduced in 2013 had not been sufficiently followed in clinical practice, and cases of serious liver injury still occurred including liver failure.

Dotarizine is a drug used in the treatment of migraine, which acts as a calcium channel blocker, and also as an antagonist at the 5HT2A receptor, and to a lesser extent at the 5HT1A and 5HT2C receptors. The anti-migraine action is thought to be due to its action as a vasodilator, but it also has some anxiolytic effects and blocks amnesia produced by electroconvulsive shock in animals.

Caroverine is a muscle-relaxing drug used in Austria and Switzerland to relieve spasms in smooth muscles, and the use in those countries was extended to aid with cerebrovascular diseases there, and eventually to treat tinnitus. It is also used to treat tinnitus in India.

Fabomotizole is an anxiolytic drug launched in Russia in the early 2000s. It produces anxiolytic and neuroprotective effects without any sedative or muscle relaxant actions. Its mechanism of action remains poorly defined however, with GABAergic, NGF- and BDNF-release-promoting, MT1 receptor agonism, MT3 receptor antagonism, and sigma agonism suggested as potential mechanisms. Fabomotizole was shown to inhibit MAO-A reversibly and there might be also some involvement with serotonin receptors. Clinical trials have shown fabomotizole to be well tolerated and reasonably effective for the treatment of anxiety.
Fimasartan is a non-peptide angiotensin II receptor antagonist (ARB) used for the treatment of hypertension and heart failure. Through oral administration, fimasartan blocks angiotensin II receptor type 1 (AT1 receptors), reducing pro-hypertensive actions of angiotensin II, such as systemic vasoconstriction and water retention by the kidneys. Concurrent administration of fimasartan with diuretic hydrochlorothiazide has shown to be safe in clinical trials. Fimasartan was approved for use in South Korea on September 9, 2010, and is available under the brand name Kanarb through Boryung Pharmaceuticals, who are presently seeking worldwide partnership.
A cerebral activator, also known as a cerebral metabolic enhancer or activator, is a type of drug that "activates" the central nervous system in the context of cerebrovascular diseases such as stroke and dementia. The term has been used specifically to describe a few Japanese drugs, such as indeloxazine and bifemelane.

Indacaterol/glycopyrronium bromide, sold under the brand name Ultibro Breezhaler among others, is a fixed-dose combination medication for inhalation consisting of the following two active ingredients:

Hypertension is a condition characterized by an elevated blood pressure in which the long term consequences include cardiovascular disease, kidney disease, adrenal gland tumors, vision impairment, memory loss, metabolic syndrome, stroke and dementia. It affects nearly 1 in 2 Americans and remains as a contributing cause of death in the United States. There are many genetic and environmental factors involved with the development of hypertension including genetics, diet, and stress.
A cerebral vasodilator is a drug which acts as a vasodilator in the brain. They are used to improve blood flow in people with cerebrovascular insufficiency and to treat neurological disorders secondary to this condition. A number of different cerebral vasodilators exist. An example is ifenprodil, which has been marketed for use as a cerebral vasodilator in France, Hong Kong, and Japan. Other examples include buphenine (nylidrin), isoxsuprine, oxyfedrine, suloctidil, and tinofedrine.

















