
Pantothenic acid (vitamin B5) is a B vitamin and an essential nutrient. All animals need pantothenic acid in order to synthesize coenzyme A (CoA), which is essential for cellular energy production and for the synthesis and degradation of proteins, carbohydrates, and fats.

β-Alanine (beta-alanine) is a naturally occurring beta amino acid, which is an amino acid in which the amino group is attached to the β-carbon instead of the more usual α-carbon for alanine (α-alanine). The IUPAC name for β-alanine is 3-aminopropanoic acid. Unlike its counterpart α-alanine, β-alanine has no stereocenter.

Panthenol (also called pantothenol) is the alcohol analog of pantothenic acid (vitamin B5), and is thus a provitamin of B5. In organisms, it is quickly oxidized to pantothenic acid. It is a viscous transparent liquid at room temperature. Panthenol is used in pharmaceutical and kids' products as a moisturizer and to hasten wound healing.
Roxatidine acetate is a specific and competitive histamine H2 receptor antagonist drug that is used to treat gastric ulcers, Zollinger–Ellison syndrome, erosive esophagitis, gastro-oesophageal reflux disease, and gastritis.

EX-597 is a fatty acid amide hydrolase inhibitor which is under development for the treatment of social anxiety disorder and post-traumatic stress disorder (PTSD).

Max Tishler (October 30, 1906 – March 18, 1989) was president of Merck Sharp and Dohme Research Laboratories where he led the research teams that synthesized ascorbic acid, riboflavin, cortisone, pyridoxine, pantothenic acid, nicotinamide, methionine, threonine, and tryptophan. He also developed the fermentation processes for actinomycin, vitamin B12, streptomycin, and penicillin. Tishler invented sulfaquinoxaline for the treatment for coccidiosis.

Phenibut, sold under the brand name Anvifen among others, is a central nervous system (CNS) depressant with anxiolytic effects, and is used to treat anxiety, insomnia, and for a variety of other indications. It is usually taken orally, but may be given intravenously.

Picamilon is a drug formed by a synthetic combination of niacin and γ-aminobutyric acid (GABA). It was developed in the Soviet Union in 1969 and further studied in both Russia and Japan as a prodrug of GABA.

Pantetheine is the cysteamine amide analog of pantothenic acid (vitamin B5). The dimer of this compound, pantethine is more commonly known, and is considered to be the most potent form of vitamin B5. Pantetheine is an intermediate in the catabolism of coenzyme A by the body.

Pazinaclone (DN-2327) is a sedative and anxiolytic drug in the cyclopyrrolone family of drugs. Some other cyclopyrrolone drugs include zopiclone and eszopiclone.
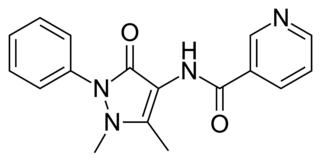
Nifenazone is a drug that has been used as an analgesic for a number of rheumatic conditions.
In enzymology, a pantoate 4-dehydrogenase (EC 1.1.1.106) is an enzyme that catalyzes the chemical reaction

γ-Amino-β-hydroxybutyric acid (GABOB), also known as β-hydroxy-γ-aminobutyric acid (β-hydroxy-GABA), sold under the brand name Gamibetal among others, is an anticonvulsant which is used for the treatment of epilepsy in Europe, Japan, and Mexico. It is a GABA analogue, or an analogue of the neurotransmitter γ-aminobutyric acid (GABA), and has been found to be an endogenous metabolite of GABA.
In enzymology, a pantothenase (EC 3.5.1.22) is an enzyme that catalyzes the chemical reaction

Lysergic acid 2-butyl amide (2-Butyllysergamide, LSB) is an analogue of LSD originally developed by Richard Pioch at Eli Lilly in the 1950s, but mostly publicised through research conducted by the team led by David E. Nichols at Purdue University. It is a structural isomer of LSD, with the two ethyl groups on the amide nitrogen having been replaced by a single sec-butyl group, joined at the 2-position. It is one of the few lysergamide derivatives to exceed the potency of LSD in animal drug discrimination assays, with the (R) isomer having an ED50 of 33nmol/kg for producing drug-appropriate responding, vs 48nmol/kg for LSD itself. The corresponding (R)-2-pentyl analogue has higher binding affinity for the 5-HT1A and 5-HT2A receptors, but is less potent in producing drug-appropriate responding, suggesting that the butyl compound has a higher efficacy at the receptor target. The drug discrimination assay for LSD in rats involves both 5-HT1A and 5-HT2A mediated components, and while lysergic acid 2-butyl amide is more potent than LSD as a 5-HT1A agonist, it is slightly less potent as a 5-HT2A agonist, and so would probably be slightly less potent than LSD as a hallucinogen in humans. The main use for this drug has been in studies of the binding site at the 5-HT2A receptor through which LSD exerts most of its pharmacological effects, with the stereoselective activity of these unsymmetric monoalkyl lysergamides foreshadowing the subsequent development of compounds such as lysergic acid 2,4-dimethylazetidide (LSZ).
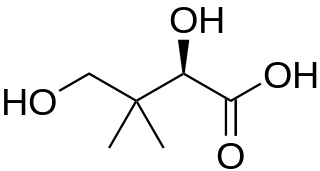
Pantoic acid is the alpha hydroxy acid with the formula HOCH2C(CH3)2CH(OH)CO2H. The compound is almost always encountered in a biological context, as an aqueous solution of its conjugate base pantoate HOCH2C(CH3)2CH(OH)CO2-. The amide of pantoic acid with β-alanine is pantothenic acid (vitamin B5), a component of coenzyme A.
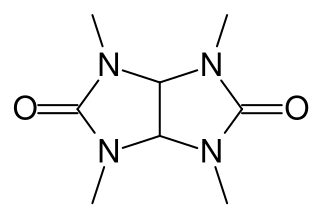
Temgicoluril, also known as tetramethylglycoluril and sold under the brand names Adaptol and Mebicar, is an anxiolytic medication produced by Latvian pharmaceutical company Olainfarm and sold in Latvia and Russia.
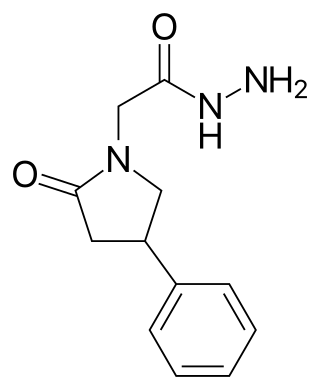
Phenylpiracetam hydrazide, also known as fonturacetam hydrazide, is a racetam that is a derivative of phenylpiracetam in which the amide group is replaced with a hydrazide group. It was first reported by a Russian research group in 1980 as part of a series of chemical compounds investigated as anticonvulsants. In an electroshock test it was found to have an ED50 of 310 mg/kg.

Tolibut, also known as 3-(p-tolyl)-4-aminobutyric acid (or β-(4-methylphenyl)-GABA), is drug that was developed in Russia. It is an analogue of γ-aminobutyric acid (GABA) and is the 4-methyl analogue of phenibut, and is also an analogue of baclofen where the 4-chloro substitution has been replaced with a 4-methyl substitution. Tolibut has been described as possessing analgesic, tranquilizing, and neuroprotective properties. It is not fully clear as to whether the drug was ever approved or used medically in Russia.

















