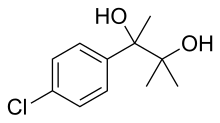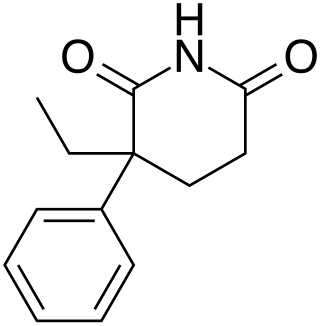
Glutethimide is a hypnotic sedative that was introduced by Ciba in 1954 as a safe alternative to barbiturates to treat insomnia. Before long, however, it had become clear that glutethimide was just as likely to cause addiction and caused similar withdrawal symptoms. Doriden was the brand-name version. Current production levels in the United States point to its use only in small-scale research. Manufacturing of the drug was discontinued in the US in 1993 and discontinued in several eastern European countries in 2006.

Amobarbital is a drug that is a barbiturate derivative. It has sedative-hypnotic properties. It is a white crystalline powder with no odor and a slightly bitter taste. It was first synthesized in Germany in 1923. It is considered a short to intermediate acting barbiturate.

Tiagabine is an anticonvulsant medication produced by Cephalon that is used in the treatment of epilepsy. The drug is also used off-label in the treatment of anxiety disorders and panic disorder.
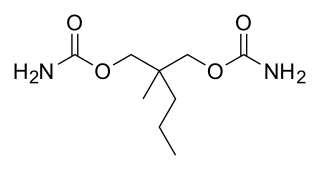
Meprobamate—marketed as Miltown by Wallace Laboratories and Equanil by Wyeth, among others—is a carbamate derivative used as an anxiolytic drug. It was the best-selling minor tranquilizer for a time, but has largely been replaced by the benzodiazepines due to their wider therapeutic index and lower incidence of serious side effects.

Phendimetrazine is a stimulant drug of the morpholine chemical class used as an appetite suppressant.
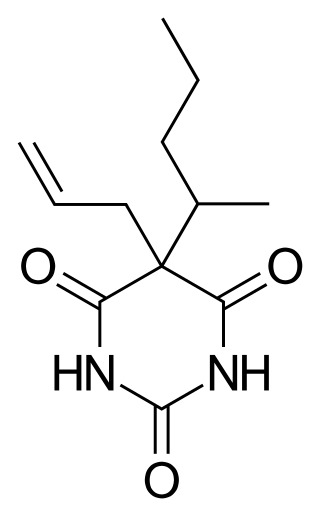
Secobarbital is a short-acting barbiturate derivative drug that was patented in 1934 in the United States. It possesses anaesthetic, anticonvulsant, anxiolytic, sedative, and hypnotic properties. In the United Kingdom, it was known as quinalbarbitone. Secobarbital is considered to be an obsolete sedative-hypnotic, and as a result, it has largely been replaced by the benzodiazepine family. Seconal was widely abused, known on the street as "red devils" or "reds". Among the barbiturates, secobarbital carries a particularly high risk of abuse and addiction, largely responsible for its falling out of use.

Clorazepate, sold under the brand name Tranxene among others, is a benzodiazepine medication. It possesses anxiolytic, anticonvulsant, sedative, hypnotic, and skeletal muscle relaxant properties. Clorazepate is an unusually long-lasting benzodiazepine and serves as a prodrug for the equally long-lasting desmethyldiazepam, which is rapidly produced as an active metabolite. Desmethyldiazepam is responsible for most of the therapeutic effects of clorazepate.

Zotepine is an atypical antipsychotic drug indicated for acute and chronic schizophrenia. It has been used in Germany since 1990 and Japan since 1982.
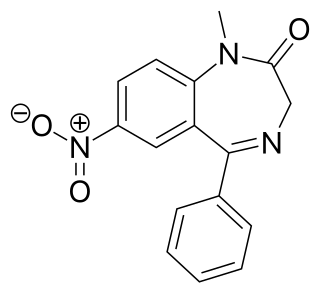
Nimetazepam is an intermediate-acting hypnotic drug which is a benzodiazepine derivative. It was first synthesized by a team at Hoffmann-La Roche in 1964. It possesses powerful hypnotic, anxiolytic, sedative, and skeletal muscle relaxant properties. Nimetazepam is also a particularly potent anticonvulsant. It is marketed in 5 mg tablets known as Erimin, which is the brand name manufactured and marketed by the large Japanese corporation Sumitomo. Japan is the sole manufacturer of nimetazepam in the world. Outside of Japan, Erimin is available in much of East and Southeast Asia and was widely prescribed for the short-term treatment of severe insomnia in patients who have difficulty falling asleep or maintaining sleep. Sumitomo has ceased manufacturing Erimin since November 2015. It is still available as a generic drug or as Lavol.

Camazepam is a benzodiazepine psychoactive drug, marketed under the brand names Albego, Limpidon and Paxor. It is the dimethyl carbamate ester of temazepam, a metabolite of diazepam. While it possesses anxiolytic, anticonvulsant, skeletal muscle relaxant and hypnotic properties it differs from other benzodiazepines in that its anxiolytic properties are particularly prominent but has comparatively limited anticonvulsant, hypnotic and skeletal muscle relaxant properties.

Benzydamine, available as the hydrochloride salt, is a locally acting nonsteroidal anti-inflammatory drug (NSAID) with local anaesthetic and analgesic properties for pain relief and anti-inflammatory treatment of inflammatory conditions of the mouth and throat. It falls under class of chemicals known as indazole.

Anthranilic acid is an aromatic acid with the formula C6H4(NH2)(CO2H) and has a sweetish taste. The molecule consists of a benzene ring, ortho-substituted with a carboxylic acid and an amine. As a result of containing both acidic and basic functional groups, the compound is amphoteric. Anthranilic acid is a white solid when pure, although commercial samples may appear yellow. The anion [C6H4(NH2)(CO2)]−, obtained by the deprotonation of anthranilic acid, is called anthranilate. Anthranilic acid was once thought to be a vitamin and was referred to as vitamin L1 in that context, but it is now known to be non-essential in human nutrition.

Phenoperidine, is an opioid analgesic which is structurally related to pethidine and is used clinically as a general anesthetic.
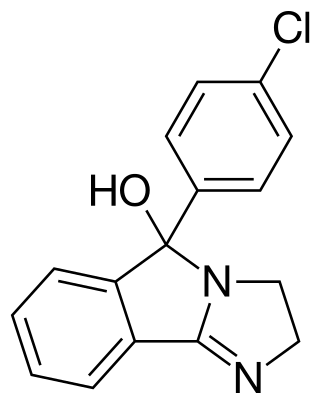
Mazindol is a stimulant drug which is used as an appetite suppressant. It was developed by Sandoz-Wander in the 1960s.

Methylpentynol is a tertiary pentynol with hypnotic/sedative and anticonvulsant effects and an exceptionally low therapeutic index. It was discovered by Bayer in 1913 and was used shortly thereafter for the treatment of insomnia, but its use was quickly phased out in response to newer drugs with far more favorable safety profiles.

Pethidinic acid is a 4-phenylpiperidine derivative that is both a metabolite of and a precursor to pethidine (meperidine). It is scheduled by UN Single Convention on Narcotic Drugs. It is a Schedule II Narcotic controlled substance in the United States and has an ACSCN of 9234. The 2014 annual manufacturing quota was 6 grams.

Morpheridine (Morpholinoethylnorpethidine) is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine). It is a strong analgesic with around 4 times the potency of pethidine, and unlike pethidine, does not cause convulsions, although it produces the standard opioid side effects such as sedation and respiratory depression.

Minaprine is a monoamine oxidase inhibitor antidepressant drug that was used in France for the treatment of depression until it was withdrawn from the market in 1996 because it caused convulsions.

Lysergic acid 2-butyl amide (2-Butyllysergamide, LSB) is an analogue of LSD originally developed by Richard Pioch at Eli Lilly in the 1950s, but mostly publicised through research conducted by the team led by David E. Nichols at Purdue University. It is a structural isomer of LSD, with the two ethyl groups on the amide nitrogen having been replaced by a single sec-butyl group, joined at the 2-position. It is one of the few lysergamide derivatives to exceed the potency of LSD in animal drug discrimination assays, with the (R) isomer having an ED50 of 33nmol/kg for producing drug-appropriate responding, vs 48nmol/kg for LSD itself. The corresponding (R)-2-pentyl analogue has higher binding affinity for the 5-HT1A and 5-HT2A receptors, but is less potent in producing drug-appropriate responding, suggesting that the butyl compound has a higher efficacy at the receptor target. The drug discrimination assay for LSD in rats involves both 5-HT1A and 5-HT2A mediated components, and while lysergic acid 2-butyl amide is more potent than LSD as a 5-HT1A agonist, it is slightly less potent as a 5-HT2A agonist, and so would probably be slightly less potent than LSD as a hallucinogen in humans. The main use for this drug has been in studies of the binding site at the 5-HT2A receptor through which LSD exerts most of its pharmacological effects, with the stereoselective activity of these unsymmetric monoalkyl lysergamides foreshadowing the subsequent development of compounds such as lysergic acid 2,4-dimethylazetidide (LSZ).

Hydroxyphenamate or oxyfenamate is a sedative and anxiolytic drug of the carbamate class which is no longer marketed in the US. Like other carbamate sedatives, it is chemically related to meprobamate (Miltown). It was introduced to the US market in 1961. The dosage for adults is 200 mg 3 to 4 times daily.