 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Chlorotri(methyl)silane | |||
| Other names Trimethylsilyl chloride Chlorotrimethylsilane TMSCl Trimethylchlorosilane TMCS | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.819 | ||
| EC Number |
| ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1298 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
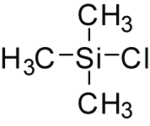
| C3H9SiCl | |||
| Molar mass | 108.64 g/mol | ||
| Appearance | Colorless liquid, fumes in moist air | ||
| Density | 0.856 g/cm3, liquid | ||
| Melting point | −40 °C (−40 °F; 233 K) | ||
| Boiling point | 57 °C (135 °F; 330 K) | ||
| Reacts | |||
| −77.36·10−6 cm3/mol | |||
| Structure | |||
| Tetrahedral at Si | |||
| Hazards | |||
| GHS labelling: | |||
     | |||
| Danger | |||
| H225, H301, H312, H314, H331, H351 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P281, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P308+P313, P310, P311, P312, P321, P322, P330, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −28 °C (−18 °F; 245 K) | ||
| 400 °C (752 °F; 673 K) | |||
| Related compounds | |||
Related halosilanes | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound (silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry.








