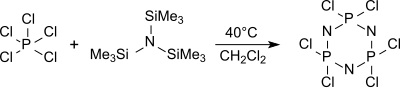The ammonium cation is a positively-charged polyatomic ion with the chemical formula NH+4 or [NH4]+. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic groups.

In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.
Cyclopropene is an organic compound with the formula C3H4. It is the simplest cycloalkene. Because the ring is highly strained, cyclopropene is difficult to prepare and highly reactive. This colorless gas has been the subject for many fundamental studies of bonding and reactivity. It does not occur naturally, but derivatives are known in some fatty acids. Derivatives of cyclopropene are used commercially to control ripening of some fruit.

Europium(III) chloride is an inorganic compound with the formula EuCl3. The anhydrous compound is a yellow solid. Being hygroscopic it rapidly absorbs water to form a white crystalline hexahydrate, EuCl3·6H2O, which is colourless. The compound is used in research.

A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si(CH3)3], which is in turn bonded to the rest of a molecule. This structural group is characterized by chemical inertness and a large molecular volume, which makes it useful in a number of applications.

Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound (silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry.
Ethylamine, also known as ethanamine, is an organic compound with the formula CH3CH2NH2. This colourless gas has a strong ammonia-like odor. It condenses just below room temperature to a liquid miscible with virtually all solvents. It is a nucleophilic base, as is typical for amines. Ethylamine is widely used in chemical industry and organic synthesis.

Organosilicon chemistry is the science of the preparation and properties of organosilicon compounds, which are organometallic compounds containing carbon–silicon bonds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.
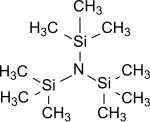
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane and HMDS) is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. An electron diffraction study shows that silicon-nitrogen bond length (173.5 pm) and Si-N-Si bond angle (125.5°) to be similar to disilazane (in which methyl groups are replaced by hydrogen atoms) suggesting that steric factors are not a factor in regulating angles in this case. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry. Additionally, HMDS is also increasingly used as molecular precursor in chemical vapor deposition techniques to deposit silicon carbonitride thin films or coatings.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.

Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula LiN(Si(CH3)3)2. It is commonly abbreviated as LiHMDS or Li(HMDS) (lithium hexamethyldisilazide - a reference to its conjugate acid HMDS) and is primarily used as a strong non-nucleophilic base and as a ligand. Like many lithium reagents, it has a tendency to aggregate and will form a cyclic trimer in the absence of coordinating species.

Tetrakis(hydroxymethyl)phosphonium chloride (THPC) is an organophosphorus compound with the chemical formula [P(CH2OH)4]Cl. The cation P(CH2OH)4+ is four-coordinate, as is typical for phosphonium salts. THPC has applications as a precursor to fire-retardant materials, as well as a microbiocide in commercial and industrial water systems.
Silylation is the introduction of one or more (usually) substituted silyl groups (R3Si) to a molecule. The process is the basis of organosilicon chemistry.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.

Metal bis(trimethylsilyl)amides are coordination complexes composed of a cationic metal with anionic bis(trimethylsilyl)amide ligands and are part of a broader category of metal amides.

Metal amides (systematic name metal azanides) are a class of coordination compounds composed of a metal center with amide ligands of the form NR2−. Amide ligands have two electron pairs available for bonding. In principle, they can be terminal or bridging. In these two examples, the dimethylamido ligands are both bridging and terminal:
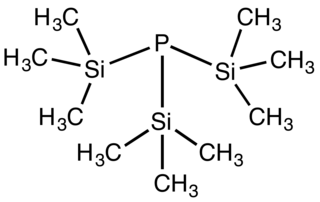
Tris(trimethylsilyl)phosphine is the organophosphorus compound with the formula P(SiMe3)3 (Me = methyl). It is a colorless liquid that ignites in air and hydrolyses readily.
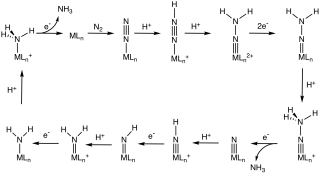
Abiological nitrogen fixation describes chemical processes that fix (react with) N2, usually with the goal of generating ammonia. The dominant technology for abiological nitrogen fixation is the Haber process, which uses an iron-based heterogeneous catalysts and H2 to convert N2 to NH3. This article focuses on homogeneous (soluble) catalysts for the same or similar conversions.