In organic chemistry, an alkane, or paraffin, is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane, where n = 1, to arbitrarily large and complex molecules, like pentacontane or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane.
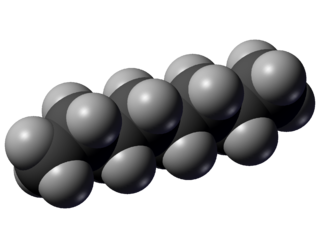
Octane is a hydrocarbon and also an alkane with the chemical formula C8H18, and the condensed structural formula CH3(CH2)6CH3. Octane has many structural isomers that differ by the location of branching in the carbon chain. One of these isomers, 2,2,4-trimethylpentane (commonly called iso-octane), is used as one of the standard values in the octane rating scale.

Heptane or n-heptane is the straight-chain alkane with the chemical formula H3C(CH2)5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale (the 100 point is 100% iso-octane). Octane number equates to the anti-knock qualities of a comparison mixture of heptane and iso-octane which is expressed as the percentage of iso-octane in heptane, and is listed on pumps for gasoline (petrol) dispensed globally.

An oil refinery or petroleum refinery is an industrial process plant where petroleum is transformed and refined into products such as gasoline (petrol), diesel fuel, asphalt base, fuel oils, heating oil, kerosene, liquefied petroleum gas and petroleum naphtha. Petrochemical feedstock like ethylene and propylene can also be produced directly by cracking crude oil without the need of using refined products of crude oil such as naphtha. The crude oil feedstock has typically been processed by an oil production plant. There is usually an oil depot at or near an oil refinery for the storage of incoming crude oil feedstock as well as bulk liquid products. In 2020, the total capacity of global refineries for crude oil was about 101.2 million barrels per day.

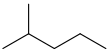
Pentane is an organic compound with the formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer, in which case pentanes refers to a mixture of them; the other two are called isopentane (methylbutane) and neopentane (dimethylpropane). Cyclopentane is not an isomer of pentane because it has only 10 hydrogen atoms where pentane has 12.

Nonane is a linear alkane hydrocarbon with the chemical formula C9H20. It is a colorless, flammable liquid, occurring primarily in the component of the petroleum distillate fraction commonly called kerosene, which is used as a heating, tractor, and jet fuel. Nonane is also used as a solvent, distillation chaser, fuel additive, and a component in biodegradable detergents. It is also a minor component of diesel fuel.

White spirit (AU, UK and Ireland) or mineral spirits (US, Canada), also known as mineral turpentine (AU/NZ/ZA), turpentine substitute, and petroleum spirits, is a petroleum-derived clear liquid used as a common organic solvent in painting. There are also terms for specific kinds of white spirit, including Stoddard solvent and solvent naphtha (petroleum). White spirit is often used as a paint thinner, or as a component thereof, though paint thinner is a broader category of solvent. Odorless mineral spirits (OMS) have been refined to remove the more toxic aromatic compounds, and are recommended for applications such as oil painting.

Ethylbenzene is an organic compound with the formula C6H5CH2CH3. It is a highly flammable, colorless liquid with an odor similar to that of gasoline. This monocyclic aromatic hydrocarbon is important in the petrochemical industry as a reaction intermediate in the production of styrene, the precursor to polystyrene, a common plastic material. In 2012, more than 99% of ethylbenzene produced was consumed in the production of styrene.

Hydrogen selenide is an inorganic compound with the formula H2Se. This hydrogen chalcogenide is the simplest and most commonly encountered hydride of selenium. H2Se is a colorless, flammable gas under standard conditions. It is the most toxic selenium compound with an exposure limit of 0.05 ppm over an 8-hour period. Even at extremely low concentrations, this compound has a very irritating smell resembling that of decayed horseradish or "leaking gas", but smells of rotten eggs at higher concentrations.
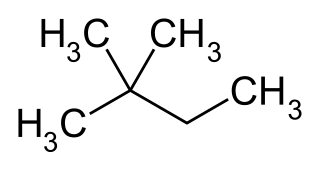
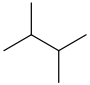
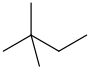
2,2-Dimethylbutane, trivially known as neohexane at William Odling's 1876 suggestion, is an organic compound with formula C6H14 or (H3C-)3-C-CH2-CH3. It is therefore an alkane, indeed the most compact and branched of the hexane isomers — the only one with a quaternary carbon and a butane (C4) backbone.

1-Butanol, also known as butan-1-ol or n-butanol, is a primary alcohol with the chemical formula C4H9OH and a linear structure. Isomers of 1-butanol are isobutanol, butan-2-ol and tert-butanol. The unmodified term butanol usually refers to the straight chain isomer.

1,2-Dichlorobenzene, or orthodichlorobenzene (ODCB), is an aryl chloride and isomer of dichlorobenzene with the formula C6H4Cl2. This colourless liquid is poorly soluble in water but miscible with most organic solvents. It is a derivative of benzene, consisting of two adjacent chlorine atoms.
1,2,4-Trichlorobenzene is an organochlorine compound, one of three isomers of trichlorobenzene. It is a derivative of benzene with three chloride substituents. It is a colorless liquid used as a solvent for a variety of compounds and materials.
Methylcyclohexane (cyclohexylmethane) is an organic compound with the molecular formula is CH3C6H11. Classified as saturated hydrocarbon, it is a colourless liquid with a faint odor.

Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon.
3-Methylpentane is a branched alkane with the molecular formula C6H14. It is a structural isomer of hexane composed of a methyl group bonded to the third carbon atom in a pentane chain. It is of similar structure to the isomeric 2-methylpentane, which has the methyl group located on the second carbon of the pentane chain.
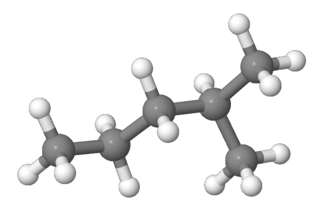
2-Methylpentane, trivially known as isohexane, is a branched-chain alkane with the molecular formula C6H14. It is a structural isomer of hexane composed of a methyl group bonded to the second carbon atom in a pentane chain.

Butane is an alkane with the formula C4H10. Butane exists as two isomers, n-butane with connectivity CH3CH2CH2CH3 and iso-butane with the formula (CH3)3CH. Both isomers are highly flammable, colorless, easily liquefied gases that quickly vaporize at room temperature and pressure. Butanes are a trace components of natural gases (NG gases). The other hydrocarbons in NG include propane, ethane, and especially methane, which are more abundant. Liquefied petroleum gas is a mixture of propane and some butanes.
Petroleum naphtha is an intermediate hydrocarbon liquid stream derived from the refining of crude oil with CAS-no 64742-48-9. It is most usually desulfurized and then catalytically reformed, which rearranges or restructures the hydrocarbon molecules in the naphtha as well as breaking some of the molecules into smaller molecules to produce a high-octane component of gasoline.
Petroleum benzine is a hydrocarbon-based solvent mixture that is classified by its physical properties rather than a specific chemical composition. This complicates distinction within the long list of petroleum distillate solvent mixtures: mineral spirits, naphtha, petroleum naptha, white gas, white spirits, turps substitute, mineral turpentine, petroleum ether, ligroin, and Stoddard solvent.