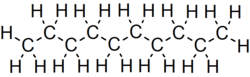 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Decane [1] | |
| Other names Decyl hydride | |
| Identifiers | |
3D model (JSmol) | |
| 1696981 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.004.262 |
| EC Number |
|
| MeSH | decane |
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 2247 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C10H22 | |
| Molar mass | 142.286 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Gasoline-like (in high concentrations) |
| Density | 0.730 g mL−1 |
| Melting point | −30.5 to −29.2 °C; −22.8 to −20.6 °F; 242.7 to 243.9 K |
| Boiling point | 173.8 to 174.4 °C; 344.7 to 345.8 °F; 446.9 to 447.5 K |
| log P | 5.802 |
| Vapor pressure | 195 Pa [2] |
Henry's law constant (kH) | 2.1 nmol Pa−1 kg−1 |
| −119.74·10−6 cm3/mol | |
| Thermal conductivity | 0.1381 W m−1 K−1 (300 K) [3] |
Refractive index (nD) | 1.411–1.412 |
| Viscosity |
|
| Thermochemistry | |
Heat capacity (C) | 315.46 J K−1 mol−1 |
Std molar entropy (S⦵298) | 425.89 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) | −302.1 – −299.9 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) | −6779.21 – −6777.45 kJ mol−1 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Flammable, moderately toxic |
| GHS labelling: | |
  | |
| Danger | |
| H226, H302, H304, H305 | |
| P301+P310, P331 | |
| NFPA 704 (fire diamond) | |
| Flash point | 46.0 °C (114.8 °F; 319.1 K) |
| 210.0 °C (410.0 °F; 483.1 K) | |
| Explosive limits | 0.8–2.6% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
|
| Safety data sheet (SDS) | hazard.com |
| Related compounds | |
Related alkanes | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Decane is an alkane hydrocarbon with the chemical formula C10H22. Although 75 structural isomers are possible for decane, the term usually refers to the normal-decane ("n-decane"), with the formula CH3(CH2)8CH3. All isomers, however, exhibit similar properties and little attention is paid to the composition. [5] These isomers are flammable liquids. Decane is present in small quantities (less than 1%) in gasoline (petrol) and kerosene. [6] [7] Like other alkanes, it is a nonpolar solvent, and does not dissolve in water, and is readily combustible. Although it is a component of fuels, it is of little importance as a chemical feedstock, unlike a handful of other alkanes. [8]

