Related Research Articles

Solder is a fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces after cooling. Metals or alloys suitable for use as solder should have a lower melting point than the pieces to be joined. The solder should also be resistant to oxidative and corrosive effects that would degrade the joint over time. Solder used in making electrical connections also needs to have favorable electrical characteristics.
Palladium hydride is palladium metal with hydrogen within its crystal lattice. Despite its name, it is not an ionic hydride but rather an alloy of palladium with metallic hydrogen that can be written PdHx. At room temperature, palladium hydrides may contain two crystalline phases, α and β. Pure α-phase exists at x < 0.017 while pure β-phase exists at x > 0.58; intermediate x values correspond to α-β mixtures.

In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to slide over each other at low stress levels and is known as glide or slip. The crystalline order is restored on either side of a glide dislocation but the atoms on one side have moved by one position. The crystalline order is not fully restored with a partial dislocation. A dislocation defines the boundary between slipped and unslipped regions of material and as a result, must either form a complete loop, intersect other dislocations or defects, or extend to the edges of the crystal. A dislocation can be characterised by the distance and direction of movement it causes to atoms which is defined by the Burgers vector. Plastic deformation of a material occurs by the creation and movement of many dislocations. The number and arrangement of dislocations influences many of the properties of materials.

Hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC), is a reduction in the ductility of a metal due to absorbed hydrogen. Hydrogen atoms are small and can permeate solid metals. Once absorbed, hydrogen lowers the stress required for cracks in the metal to initiate and propagate, resulting in embrittlement. Hydrogen embrittlement occurs most notably in steels, as well as in iron, nickel, titanium, cobalt, and their alloys. Copper, aluminium, and stainless steels are less susceptible to hydrogen embrittlement.

An intermetallic is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic elements. Intermetallics are generally hard and brittle, with good high-temperature mechanical properties. They can be classified as stoichiometric or nonstoichiometic intermetallic compounds.
Nascent hydrogen is an outdated concept in organic chemistry that was once invoked to explain dissolving-metal reactions, such as the Clemmensen reduction and the Bouveault–Blanc reduction. Since organic compounds do not react with H2, a special state of hydrogen was postulated. It is now understood that dissolving-metal reactions occur at the metal surface, and the concept of nascent hydrogen has been discredited in organic chemistry. However, the formation of atomic hydrogen is largely invoked in inorganic chemistry and corrosion sciences to explain hydrogen embrittlement in metals exposed to electrolysis and anaerobic corrosion (e.g., dissolution of zinc in strong acids (HCl) and aluminium in strong bases (NaOH). The mechanism of hydrogen embrittlement was first proposed by Johnson (1875). The inability of hydrogen atoms to react with organic reagents in organic solvents does not exclude the transient formation of hydrogen atoms capable to immediately diffuse into the crystal lattice of common metals (steel, titanium) different from these of the platinoid group (Pt, Pd, Rh, Ru, Ni) which are well known to dissociate molecular dihydrogen (H2) into atomic hydrogen.

A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Key characteristics of a superalloy include mechanical strength, thermal creep deformation resistance, surface stability, and corrosion and oxidation resistance.

A reactor pressure vessel (RPV) in a nuclear power plant is the pressure vessel containing the nuclear reactor coolant, core shroud, and the reactor core.
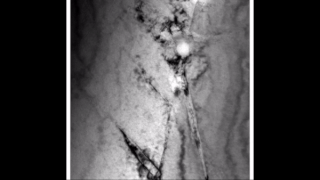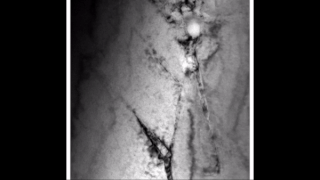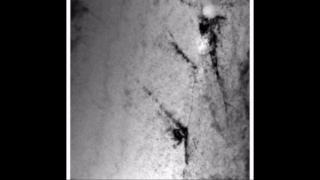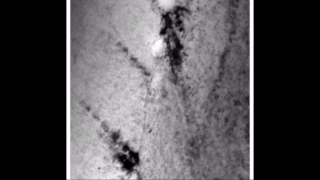
Zirconium hydride describes an alloy made by combining zirconium and hydrogen. Hydrogen acts as a hardening agent, preventing dislocations in the zirconium atom crystal lattice from sliding past one another. Varying the amount of hydrogen and the form of its presence in the zirconium hydride controls qualities such as the hardness, ductility, and tensile strength of the resulting zirconium hydride. Zirconium hydride with increased hydrogen content can be made harder and stronger than zirconium, but such zirconium hydride is also less ductile than zirconium.
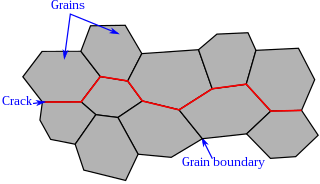
Intergranular fracture, intergranular cracking or intergranular embrittlement occurs when a crack propagates along the grain boundaries of a material, usually when these grain boundaries are weakened. The more commonly seen transgranular fracture, occurs when the crack grows through the material grains. As an analogy, in a wall of bricks, intergranular fracture would correspond to a fracture that takes place in the mortar that keeps the bricks together.
Hydrogen damage is the generic name given to a large number of metal degradation processes due to interaction with hydrogen atoms. Note that molecular gaseous hydrogen does not have the same effect as atoms or ions released into solid solution in the metal.

Embrittlement is a significant decrease of ductility of a material, which makes the material brittle. Embrittlement is used to describe any phenomena where the environment compromises a stressed material's mechanical performance, such as temperature or environmental composition. This is oftentimes undesirable as brittle fracture occurs quicker and can much more easily propagate than ductile fracture, leading to complete failure of the equipment. Various materials have different mechanisms of embrittlement, therefore it can manifest in a variety of ways, from slow crack growth to a reduction of tensile ductility and toughness.

Titanium hydride normally refers to the inorganic compound TiH2 and related nonstoichiometric materials. It is commercially available as a stable grey/black powder, which is used as an additive in the production of Alnico sintered magnets, in the sintering of powdered metals, the production of metal foam, the production of powdered titanium metal and in pyrotechnics.
Low hydrogen annealing, commonly known as "baking" is a heat treatment in metallurgy for the reduction or elimination of hydrogen in a material to prevent hydrogen embrittlement. Hydrogen embrittlement is the hydrogen-induced cracking of metals, particularly steel which results in degraded mechanical properties such as plasticity, ductility and fracture toughness at low temperature. Low hydrogen annealing is called a de-embrittlement process. Low hydrogen annealing is an effective method compared to alternatives such as electroplating the material with zinc to provide a barrier for hydrogen ingress which results in coating defects.
Uranium hydride, also called uranium trihydride (UH3), is an inorganic compound and a hydride of uranium.
Chromium hydrides are compounds of chromium and hydrogen, and possibly other elements. Intermetallic compounds with not-quite-stoichometric quantities of hydrogen exist, as well as highly reactive molecules. When present at low concentrations, hydrogen and certain other elements alloyed with chromium act as softening agents that enables the movement of dislocations that otherwise not occur in the crystal lattices of chromium atoms.
Iron(II) hydride, systematically named iron dihydride and poly(dihydridoiron) is solid inorganic compound with the chemical formula (FeH
2)
n (also written ([FeH
2])n or FeH
2). ). It is kinetically unstable at ambient temperature, and as such, little is known about its bulk properties. However, it is known as a black, amorphous powder, which was synthesised for the first time in 2014.

Iron–hydrogen alloy, also known as iron hydride, is an alloy of iron and hydrogen and other elements. Because of its lability when removed from a hydrogen atmosphere, it has no uses as a structural material.
High temperature hydrogen attack (HTHA), also called hot hydrogen attack or methane reaction, is a problem which concerns steels operating at elevated temperatures (typically above 400 °C (752 °F)) in hydrogen-rich atmospheres, such as refineries, petrochemical and other chemical facilities and, possibly, high pressure steam boilers. It is not to be confused with hydrogen embrittlement.
Hydrogen compounds are compounds containg the element hydrogen. In these compounds, hydrogen can form in the +1 and -1 oxidation states. Hydrogen can form compounds both ionically and in covalent substances. It is a part of many organic compounds such as hydrocarbons as well as water and other organic substances. The H+ ion is often called a proton because it has one proton and no electrons, although the proton does not move freely. Brønsted–Lowry acids are capable of donating H+ ions to bases.
References
- ↑ Stanislaw M. Filipek, Izabella Grzegory, Janusz Lipkowski, Stanislaw Sieniutycz. "In Memoriam: Professor Bogdan Baranowski". researchgate.net. Retrieved 8 November 2023.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Xu, Xuejun; Mao Wen; Zhong Hu; Seiji Fukuyama; Kiyoshi Yokogawa (2002). "Atomistic process on hydrogen embrittlement of a single crystal of nickel by the embedded atom method". Computational Materials Science. 23 (1–4). Elsevier: 131–138. doi:10.1016/s0927-0256(01)00217-8.
- ↑ Xu, Xuejen; Mao Wen; Seiji Fukuyama; Kioshi Yokogawa (2001). "Simulation of Hydrogen Embrittlement at Crack Tip in Nickel Single Crystal by Embedded Atom Method". Materials Transactions. 42 (11): 2283–2289. doi: 10.2320/matertrans.42.2283 . ISSN 1345-9678.
- 1 2 3 4 Shan, Junjun (11 November 2009). On the formation and decomposition of a thin NiHx layer on Ni(111) (PDF). Leiden: Universiteit Leiden. p. 94. ISBN 9789085704171 . Retrieved 11 February 2013.
{{cite book}}:|work=ignored (help) - 1 2 Takano, Noriyuki; Shinichirou Kaida (2012). "Crack Initiation by Cathodic Hydrogen Charging in Nickel Single Crystal". ISIJ International. 52 (2): 263–266. doi: 10.2355/isijinternational.52.263 . S2CID 135948644.
- 1 2 Travares, S. S. M.; A. Lafuente; S. Miraglia; D. Fruchart; S. Pairis (2003). "SEM Characterization of Hydrogenated Nickel". Acta Microscopia. 12 (1).
- ↑ Eberhardt, N. A.; Guan, H. (2016). "Nickel Hydride Complexes". Chemical Reviews. 116 (15): 8373–8426. doi:10.1021/acs.chemrev.6b00259. PMID 27437790.