
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature.

Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to chemical compounds large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.
In chemistry, catenation is the bonding of atoms of the same element into a series, called a chain. A chain or a ring shape may be open if its ends are not bonded to each other, or closed if they are bonded in a ring. The words to catenate and catenation reflect the Latin root catena, "chain".
Hydrogen selenide is an inorganic compound with the formula H2Se. This hydrogen chalcogenide is the simplest and most commonly encountered hydride of selenium. H2Se is a colorless, flammable gas under standard conditions. It is the most toxic selenium compound with an exposure limit of 0.05 ppm over an 8-hour period. Even at extremely low concentrations, this compound has a very irritating smell resembling that of decayed horseradish or 'leaking gas', but smells of rotten eggs at higher concentrations.
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, its derivative sodium selenate is used in the production of glass and animal feeds.
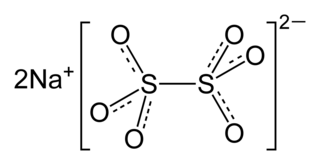
Sodium dithionate Na2S2O6 is an important compound for inorganic chemistry. It is also known under names disodium dithionate, sodium hyposulfate, and sodium metabisulfate. The sulfur can be considered to be in its +5 oxidation state.

Disulfur dichloride is the inorganic compound of sulfur and chlorine with the formula S2Cl2.
A polysulfane is a chemical compound of formula H2Sn, where n > 1. Polysulfanes consist of unbranched chains of sulfur atoms terminated with hydrogen atoms. Compounds containing 2 – 8 concatenated sulfur atoms have been isolated, longer chain compounds have been detected, but only in solution. H2S2 is colourless, higher members are yellow with the colour increasing with the sulfur content. Even a trace of alkali will cause chemical decomposition, and containers need to be treated with acid to remove any traces of alkali. In the chemical literature the term polysulfanes is sometimes used for compounds containing −(S)n−, e.g. organic polysulfanes R1−(S)n−R2.
Thiosulfuric acid is the inorganic compound with the formula H2S2O3. It has attracted academic interest as a simple, easily accessed compound that is labile. It has few practical uses.

The element sulfur exists as many allotropes. In number of allotropes, sulfur is second only to carbon. In addition to the allotropes, each allotrope often exists in polymorphs delineated by Greek prefixes.

Bismuth(III) sulfide is a chemical compound of bismuth and sulfur. It occurs in nature as the mineral bismuthinite.

Hydrogen disulfide is the inorganic compound with the formula H2S2. This hydrogen chalcogenide is a pale yellow volatile liquid with a camphor-like odor. It decomposes readily to hydrogen sulfide (H2S) and elemental sulfur.
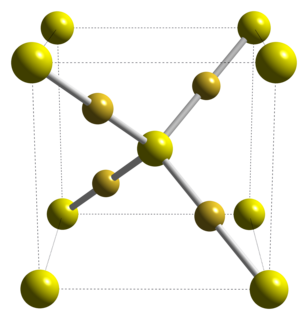
Gold(I) sulfide is the inorganic compound with the formula Au2S. It is the principal sulfide of gold. It decomposes to gold metal and elemental sulfur, illustrating the "nobility" of gold.

Polythionic acid is an oxoacid which has a straight chain of sulfur atoms and has the chemical formula Sn(SO3H)2 (n > 2). Trithionic acid (H2S3O6), tetrathionic acid (H2S4O6) are simple examples. They are the conjugate acids of polythionates. The compounds of n < 80 are expected to exist, and those of n < 20 have already been synthesized. Dithionic acid (H2S2O6) does not belong to the polythionic acids due to strongly different properties.

Sodium polysulfide is a general term for salts with the formula Na2Sx, where x = 2 to 5. The species Sx2−, called polysulfide anions, include disulfide (S22−), trisulfide (S32−), tetrasulfide (S42−), and pentasulfide (S52−). In principle, but not in practice, the chain lengths could be longer. The salts are dark red solids that dissolve in water to give highly alkaline and corrosive solutions. In air, these salts oxidize, and they evolve hydrogen sulfide by hydrolysis.

Sulfur dibromide is the chemical compound with the formula SBr2. It is a toxic gas.

Thiosulfurous acid (HS−S(=O)−OH) is a hypothetical compound with the formula S2(OH)2. Attempted synthesis leads to polymers. It is a low oxidation state (+1) sulfur acid. It is the equivalent acid for disulfur monoxide. Salts derived from thiosulfurous acid, which are also unknown, are named "thiosulfites" or "sulfurothioites". The ion is S=SO2−
2.
Hydrogen chalcogenides are binary compounds of hydrogen with chalcogen atoms. Water, the first chemical compound in this series, contains one oxygen atom and two hydrogen atoms, and is the most common compound on the Earth's surface.
Tungsten trisulfide is an inorganic compound of tungsten and sulfur with the chemical formula WS3. The compound looks like chocolate-brown powder.
Polonium sulfide is an inorganic compound of polonium and sulfur with the chemical formula PoS. The compound is radioactive, forms black crystals.












