Related Research Articles

In chemistry, a dihydrogen bond is a kind of hydrogen bond, an interaction between a metal hydride bond and an OH or NH group or other proton donor. With a van der Waals radius of 1.2 Å, hydrogen atoms do not usually approach other hydrogen atoms closer than 2.4 Å. Close approaches near 1.8 Å, are, however, characteristic of dihydrogen bonding.
Borderline hydrides typically refer to hydrides formed of hydrogen and elements of the periodic table in group 11 and group 12 and indium (In) and thallium (Tl). These compounds have properties intermediate between covalent hydrides and saline hydrides. Hydrides are chemical compounds that contain a metal and hydrogen acting as a negative ion.

Aluminium hydride is an inorganic compound with the formula AlH3. Alane and its derivatives are part of a family of common reducing reagents in organic synthesis based around group 13 hydrides. In solution—typically in etherial solvents such tetrahydrofuran or diethyl ether—aluminium hydride forms complexes with Lewis bases, and reacts selectively with particular organic functional groups, and although it is not a reagent of choice, it can react with carbon-carbon multiple bonds. Given its density, and with hydrogen content on the order of 10% by weight, some forms of alane are, as of 2016, active candidates for storing hydrogen and so for power generation in fuel cell applications, including electric vehicles. As of 2006 it was noted that further research was required to identify an efficient, economical way to reverse the process, regenerating alane from spent aluminium product.
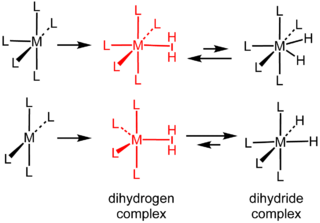
Dihydrogen complexes are coordination complexes containing intact H2 as a ligand. They are a subset of sigma complexes. The prototypical complex is W(CO)3(PCy3)2(H2). This class of compounds represent intermediates in metal-catalyzed reactions involving hydrogen. Hundreds of dihydrogen complexes have been reported. Most examples are cationic transition metals complexes with octahedral geometry.
Transition metal hydrides are chemical compounds containing a transition metal bonded to hydrogen. Most transition metals form hydride complexes and some are significant in various catalytic and synthetic reactions. The term "hydride" is used loosely: some of them are acidic (e.g., H2Fe(CO)4), whereas some others are hydridic, having H−-like character (e.g., ZnH2).

Magnesium hydride is the chemical compound with the molecular formula MgH2. It contains 7.66% by weight of hydrogen and has been studied as a potential hydrogen storage medium.
Zinc hydride is an inorganic compound with the chemical formula ZnH2. It is a white, odourless solid which slowly decomposes into its elements at room temperature; despite this it is the most stable of the binary first row transition metal hydrides. A variety of coordination compounds containing Zn–H bonds are used as reducing agents, but ZnH2 itself has no common applications.
Binary compounds of hydrogen are binary chemical compounds containing just hydrogen and one other chemical element. By convention all binary hydrogen compounds are called hydrides even when the hydrogen atom in it is not an anion. These hydrogen compounds can be grouped into several types.
Cadmium hydride is an inorganic compound with the chemical formula (CdH
2)
n. It is a solid, known only as a thermally unstable, insoluble white powder.
Titanium(IV) hydride is an inorganic compound with the empirical chemical formula TiH
4. It has not yet been obtained in bulk, hence its bulk properties remain unknown. However, molecular titanium(IV) hydride has been isolated in solid gas matrices. The molecular form is a colourless gas, and very unstable toward thermal decomposition. As such the compound is not well characterised, although many of its properties have been calculated via computational chemistry.

An iron hydride is a chemical system which contains iron and hydrogen in some associated form.

Mercury(II) hydride is an inorganic compound with the chemical formula HgH
2. It is both thermodynamically and kinetically unstable at ambient temperature, and as such, little is known about its bulk properties. However, it known as a white, crystalline solid, which is kinetically stable at temperatures below −125 °C (−193 °F), which was synthesised for the first time in 1951.

Chromium(I) hydride, systematically named chromium hydride, is an inorganic compound with the chemical formula (CrH)
n. It occurs naturally in some kinds of stars where it has been detected by its spectrum. However, molecular chromium(I) hydride with the formula CrH has been isolated in solid gas matrices. The molecular hydride is very reactive. As such the compound is not well characterised, although many of its properties have been calculated via computational chemistry.
Chromium(II) hydride, systematically named chromium dihydride and poly(dihydridochromium) is pale brown solid inorganic compound with the chemical formula (CrH2)n. Although it is thermodynamically unstable toward decomposition at ambient temperatures, it is kinetically metastable.

Iron(I) hydride, systematically named iron hydride and poly(hydridoiron) is a solid inorganic compound with the chemical formula (FeH)
n (also written ([FeH])
n or FeH). It is both thermodynamically and kinetically unstable toward decomposition at ambient temperature, and as such, little is known about its bulk properties.
Iron(II) hydride, systematically named iron dihydride and poly(dihydridoiron) is solid inorganic compound with the chemical formula (FeH
2)
n (also written ([FeH
2])n or FeH
2). ). It is kinetically unstable at ambient temperature, and as such, little is known about its bulk properties. However, it is known as a black, amorphous powder, which was synthesised for the first time in 2014.
Borane, also known as borine, is an unstable and highly reactive molecule with the chemical formula BH
3. The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated the likely existence of the borane molecule. However, the molecular species BH3 is a very strong Lewis acid. Consequently, it is highly reactive and can only be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen. It normally dimerizes to diborane in the absence of other chemicals.

Magnesium monohydride is a molecular gas with formula MgH that exists at high temperatures, such as the atmospheres of the Sun and stars. It was originally known as magnesium hydride, although that name is now more commonly used when referring to the similar chemical magnesium dihydride.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.
The inorganic imides are compounds containing an ion composed of nitrogen bonded to hydrogen with formula HN2−. Organic imides have the NH group, and two single or one double covalent bond to other atoms. The imides are related to the inorganic amides (H2N−), the nitrides (N3−) and the nitridohydrides (N3−•H−).
References
- ↑ Souter, Philip F.; Kushto, Gary P.; Andrews, Lester; Neurock, Matthew (1997). "Experimental and Theoretical Evidence for the Formation of Several Uranium Hydride Molecules". Journal of the American Chemical Society. 119 (7): 1682–1687. doi:10.1021/ja9630809.
- ↑ Raab, Juraj; Lindh, Roland H.; Wang, Xuefeng; Andrews, Lester; Gagliardi, Laura (2007). "A Combined Experimental and Theoretical Study of Uranium Polyhydrides with New Evidence for the Large Complex UH4(H2)6". The Journal of Physical Chemistry A. 111 (28): 6383–6387. Bibcode:2007JPCA..111.6383R. doi:10.1021/jp0713007. PMID 17530832.