
Chloryl fluoride is the chemical compound with the formula ClO2F. It is commonly encountered as side-product in reactions of chlorine fluorides with oxygen sources. It is the acyl fluoride of chloric acid.
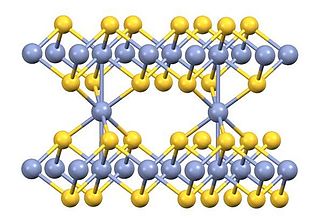
Chromium(III) sulfide is the inorganic compound with the formula Cr2S3. It is a brown-black solid. Chromium sulfides are usually nonstoichiometric compounds, with formulas ranging from CrS to Cr0.67S (corresponding to Cr2S3).

Yttrium phosphate, YPO4, is the phosphate salt of yttrium. It occurs in nature as minerals xenotime and weinschenkite.

Ammonium hexafluoroaluminate is an inorganic compound with the chemical formula of (NH4)3[AlF6]. It is a white solid. Upon heating, it converts to aluminium trifluoride, a reaction that releases hydrogen fluoride. It has also been used as a precursor to zeolites.

Uranium triiodide is an inorganic compound with the chemical formula UI3. It is a black solid that is soluble in water.
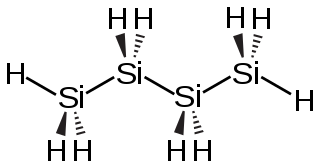
Tetrasilane is a silane with the structure formula SiH3–(SiH2)2–SiH3. It is the silane analog of butane.

Promethium(III) chloride is a chemical compound of promethium and chlorine with the formula PmCl3. It is an ionic, water soluble, crystalline salt that glows in the dark with a pale blue or green light due to promethium's intense radioactivity.
Gallium(I) oxide, digallium monoxide or gallium suboxide is an inorganic compound with the formula Ga2O.

Promethium(III) fluoride or promethium trifluoride is a salt of promethium and fluorine with the formula PmF3.
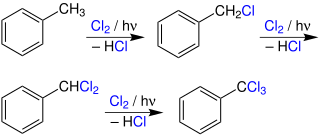
Photochlorination is a chlorination reaction that is initiated by light. Usually a C-H bond is converted to a C-Cl bond. Photochlorination is carried out on an industrial scale. The process is exothermic and proceeds as a chain reaction initiated by the homolytic cleavage of molecular chlorine into chlorine radicals by ultraviolet radiation. Many chlorinated solvents are produced in this way.
Samarium(II) fluoride is one of fluorides of samarium with a chemical formula SmF2. The compound crystalizes in the fluorite structure, and is significantly nonstoichiometric. Along with Europium(II) fluoride and Ytterbium(II) fluoride, it is one of three known rare earth difluorides, the rest are unstable.

Cerium(IV) fluoride is an inorganic compound with a chemical formula CeF4. It is a strong oxidant that appears as a white crystalline material. Cerium(IV) fluoride has an anhydrous form and a monohydrate form.
Europium(II) fluoride is an inorganic compound with a chemical formula EuF2. It was first synthesized in 1937.

Rubidium sulfide is an inorganic compound and a salt with the chemical formula Rb2S. It is a white solid with similar properties to other alkali metal sulfides.

Neptunium(III) chloride or neptunium trichloride is an inorganic compound with a chemical formula NpCl3. This salt is strongly radioactive.

Thiothionyl fluoride is a chemical compound of fluorine and sulfur, with the chemical formula S=SF2. It is an isomer of disulfur difluoride (difluorodisulfane) F−S−S−F.
Tungsten(III) iodide or tungsten triiodide is a chemical compound of tungsten and iodine with the formula WI3.
Tetraoxygen difluoride is an inorganic chemical compound of oxygen, belonging to the family of oxygen fluorides. It consists of two O2F units bound together with a weak O-O bond, and is the dimer of the O2F radical.

Chromium(II) sulfide is an inorganic compound of chromium and sulfur with the chemical formula CrS. The compound forms black hexagonal crystals, insoluble in water.
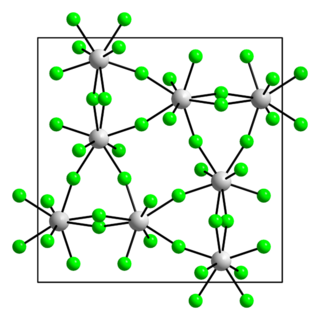
Protactinium(V) fluoride is a fluoride of protactinium with the chemical formula PaF5.

















