
The noble gases are the naturally occurring members of group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Under standard conditions, these elements are odorless, colorless, monatomic gases with very low chemical reactivity and cryogenic boiling points.
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.

Xenon tetrafluoride is a chemical compound with chemical formula XeF
4. It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine:
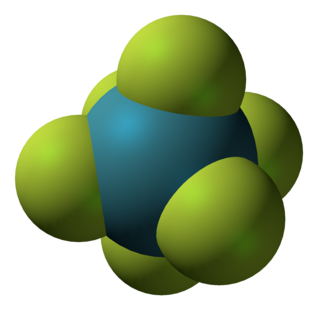
Xenon hexafluoride is a noble gas compound with the formula XeF6. It is one of the three binary fluorides of xenon that have been studied experimentally, the other two being XeF2 and XeF4. All known are exergonic and stable at normal temperatures. XeF6 is the strongest fluorinating agent of the series. It is a colorless solid that readily sublimes into intensely yellow vapors.

Hydrogen fluoride (fluorane) is an inorganic compound with chemical formula HF. It is a very poisonous, colorless gas or liquid that dissolves in water to yield an aqueous solution termed hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils at near room temperature, much higher than other hydrogen halides.
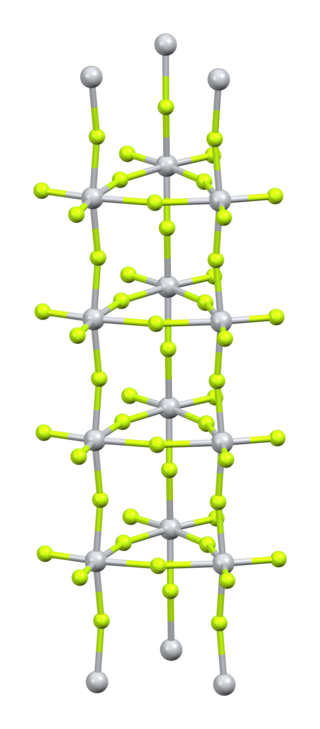
Titanium(IV) fluoride is the inorganic compound with the formula TiF4. It is a white hygroscopic solid. In contrast to the other tetrahalides of titanium, it adopts a polymeric structure. In common with the other tetrahalides, TiF4 is a strong Lewis acid.

Krypton difluoride, KrF2 is a chemical compound of krypton and fluorine. It was the first compound of krypton discovered. It is a volatile, colourless solid at room temperature. The structure of the KrF2 molecule is linear, with Kr−F distances of 188.9 pm. It reacts with strong Lewis acids to form salts of the KrF+ and Kr
2F+
3 cations.

Manganese tetrafluoride, MnF4, is the highest fluoride of manganese. It is a powerful oxidizing agent and is used as a means of purifying elemental fluorine.
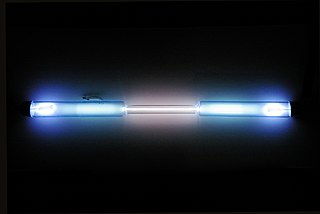
Krypton is a chemical element; it has symbol Kr and atomic number 36. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts in the atmosphere and is often used with other rare gases in fluorescent lamps. Krypton is chemically inert.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.

Palladium (IV) fluoride, also known as palladium tetrafluoride, is the chemical compound of palladium and fluorine with the chemical formula PdF4. The palladium atoms in PdF4 are in the +4 oxidation state.
A tetrafluoride is a chemical compound with four fluorines in its formula.
Nitrogen pentafluoride is a theoretical compound of nitrogen and fluorine with the chemical formula NF5. It is hypothesized to exist based on the existence of the pentafluorides of the atoms below nitrogen in the periodic table, such as phosphorus pentafluoride. Theoretical models of the nitrogen pentafluoride molecule are either a trigonal bipyramidal covalently bound molecule with symmetry group D3h, or [NF4]+F−, which would be an ionic solid.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
Lucia V. Streng was a Russian Empire-born American chemist. She spent much of her career studying the noble gases and their properties, successfully synthesizing krypton difluoride. She and her husband, Alex G. Streng, both held positions at Temple University.

Radon compounds are chemical compounds formed by the element radon (Rn). Radon is a noble gas, i.e. a zero-valence element, and is chemically not very reactive. The 3.8-day half-life of radon-222 makes it useful in physical sciences as a natural tracer. Because radon is a gas under normal circumstances, and its decay-chain parents are not, it can readily be extracted from them for research.

Krypton hexafluoride is an inorganic chemical compound of krypton and fluorine with the chemical formula KrF6. It is still a hypothetical compound. Calculations indicate it is unstable.
Palladium hexafluoride is an inorganic chemical compound of palladium metal and fluorine with the chemical formula PdF6. It is reported to be a still hypothetical compound. This is one of many palladium fluorides.
Nickel tetrafluoride is an inorganic compound with a chemical formula NiF4.











